Biological degradable PES graft polyphosphonitrile copolymer and its preparation
A degradable, copolymer technology, applied in the field of biodegradable polyester graft polyphosphazene copolymer and its preparation, can solve the problems of slow degradation rate and slow degradation rate of the graft copolymer, and reduce the sterility Inflammation, excellent biodegradability, the effect of a wide range of biomedical applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
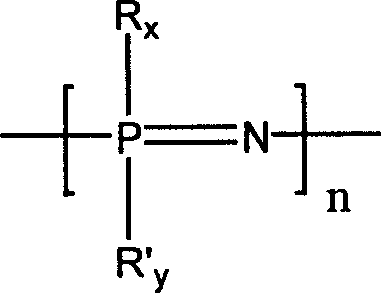
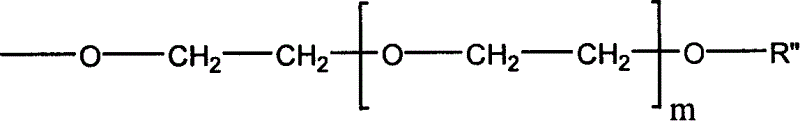
[0042] Dissolve 0.58 g of linear polydichlorophosphazene (the degree of polymerization is about 450, and the molecular weight is 50,000 to 60,000) obtained by ring-opening polymerization of hexachlorophosphazene ring trimer in 10 ml of dry tetrahydrofuran, and then slowly pour into it Add dropwise 50 ml of a tetrahydrofuran solution containing 7.0 g of sodium methoxypolyethylene glycol (taking polyether with a molecular weight of 350 as an example), and reflux at 60° C. for 24 hours. The generated sodium chloride was removed by filtration, and then the tetrahydrofuran solution was precipitated with n-hexane to obtain a methoxypolyethylene glycol-substituted polyphosphazene (PMEP-1) elastomer.
[0043] Dissolve 7.45 g of the above-mentioned elastomer in 50 ml of chloroform, then add 2.0 g of iodotrimethylsilane to it, heat up to 30-35° C. and react for 3 days, then distill off the solvent under reduced pressure to dryness. Then the resultant was dissolved in tetrahydrofuran, 5:...
Embodiment 2
[0046] Dissolve 1.16 g of linear polydichlorophosphazene (polymerization degree about 1000, molecular weight about 100,000) obtained by ring-opening polymerization of hexachlorophosphazene ring trimer in 20 ml of dry tetrahydrofuran, and then slowly drop it Add 50 ml of tetrahydrofuran solution containing 4.0 g of sodium methoxyethoxide, and reflux at 60°C for 24 hours. The generated sodium chloride was removed by filtration, and then the tetrahydrofuran solution was precipitated with n-hexane to obtain a methoxyethoxy-substituted polyphosphazene (PMEP-2) elastomer.
[0047] Dissolve 3.90 g of the above-mentioned elastomer in 40 ml of dichloromethane, then add 4.0 g of iodotrimethylsilane to it, heat up to 30-35° C. and react for 3 days, then distill off the solvent under reduced pressure to dryness. Then dissolve the resultant in dioxane, add 3:1 (tetrahydrofuran / water, v / v) water to it, hydrolyze at room temperature for 1 hour, and remove the solvent and water to obtain ethy...
Embodiment 3
[0050] Dissolve PMEP-2 (3.90g) prepared according to Example 2 in 40ml of chloroform, then add 2.40g of iodotrimethylsilane to it, heat up to 30-35°C and react for 2 days, evaporate the solvent under reduced pressure to dryness . Then the resultant was dissolved in tetrahydrofuran, 5:1 (tetrahydrofuran / water, v / v) water was added thereto, hydrolyzed at room temperature for 1 hour, and after solvent and water were removed, 30% ethylene glycol substituted with a hydroxyl group at the end was obtained. Methoxyethoxy 70% substituted polyphosphazenes (PEP-3).
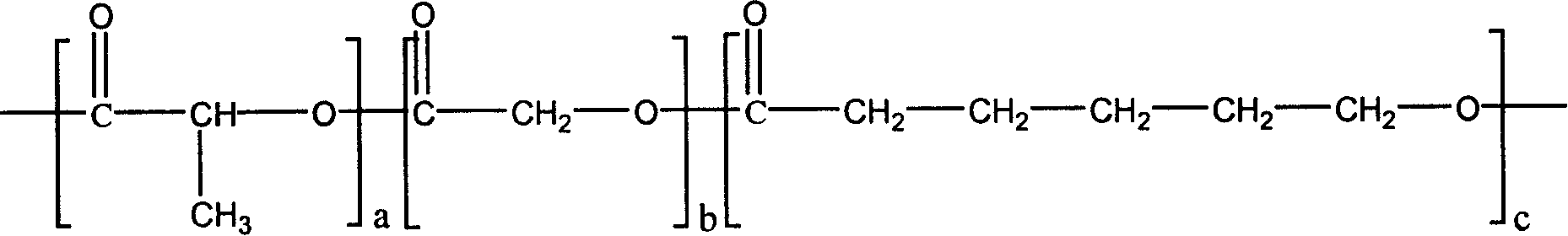
[0051] PEP-3 (1.87g) and D, L-lactide 4.33g, under the protection of an inert atmosphere (argon or nitrogen), add 0.006g of stannous isooctanoate, and then under vacuum conditions (below 15Pa) React at 140°C for 12 hours to obtain poly(D,L-lactide) grafted polyphosphazene. The graft ratio of poly(D,L-lactide) is 30%, and the average segment length is 10 lactic acid units.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of grafting | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com