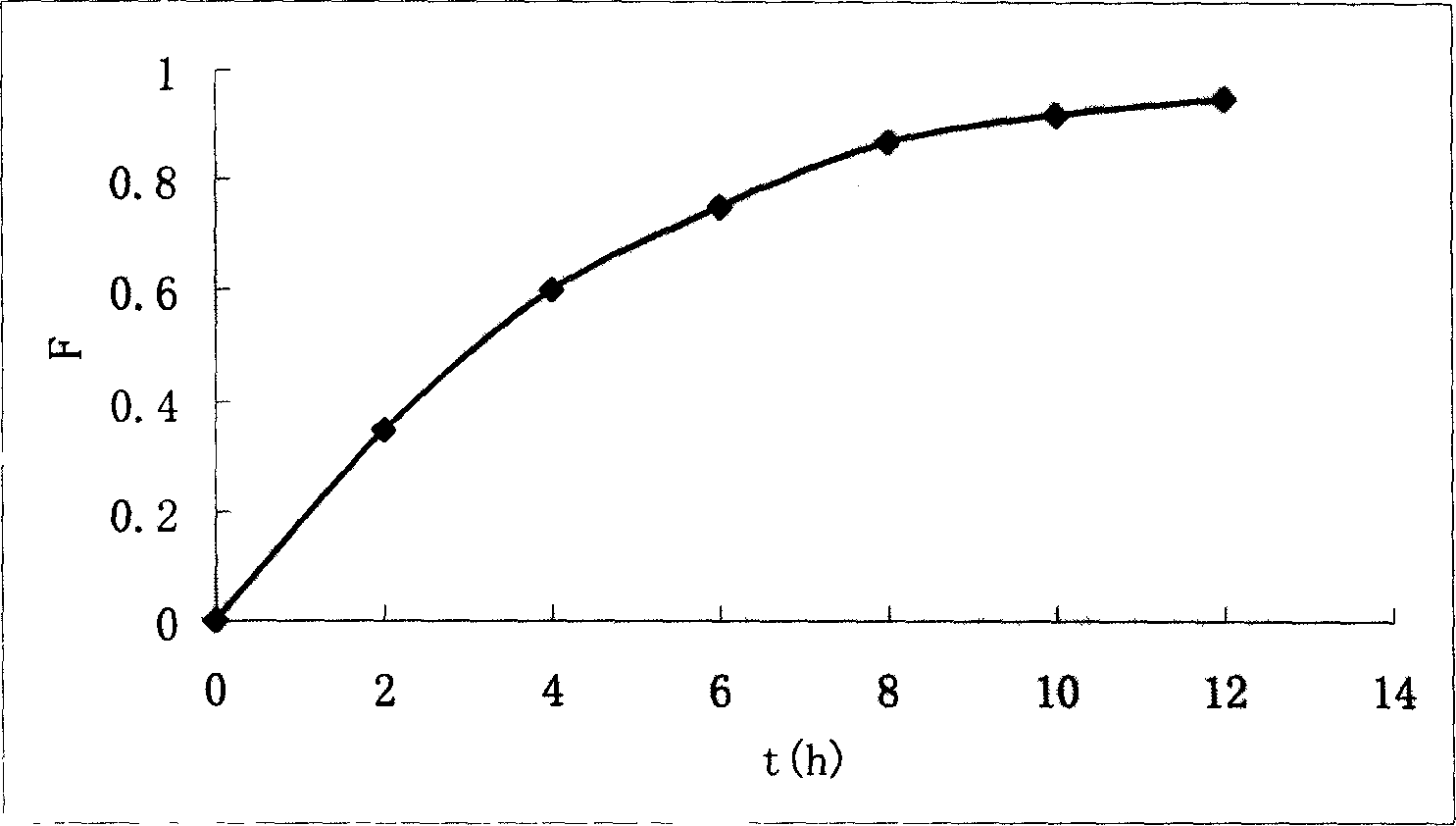
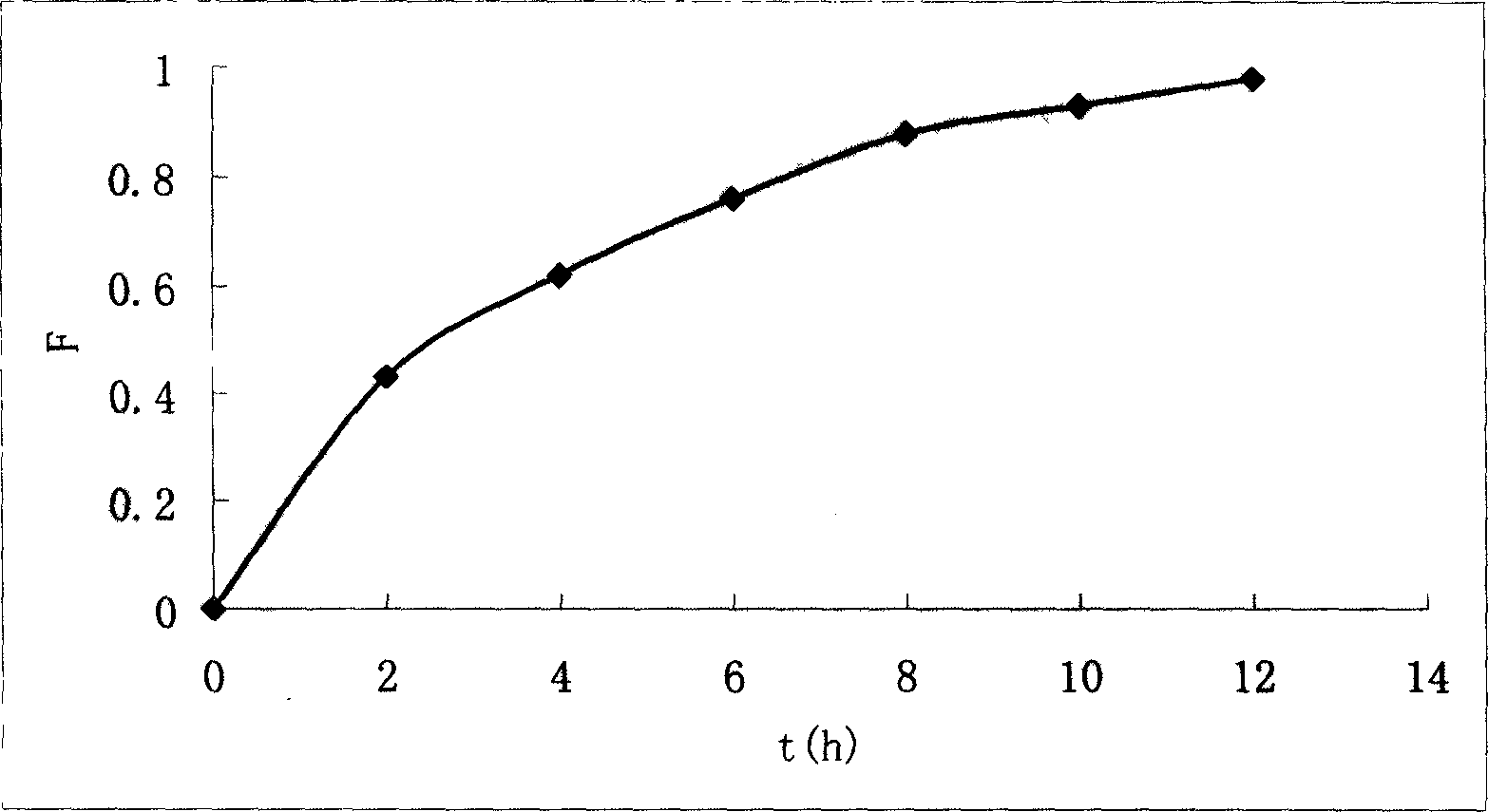
Medicinal resin biological adhering slow-releasing liquid prepn. contg. aciclovir, and its prepn. method
A liquid preparation and bioadhesion technology, applied in the field of medicine, to achieve the effects of feasible process, promotion of absorption and easy production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Acyclovir: 600mg;
[0035] Amberlite IRP69: 500mg;
[0036] Hypromellose K15M 50mg
[0037] PEG4000: 200mg;
[0038] PEG400: 5mg;
[0039]Diethyl phthalate: 5mg;
[0040] EC (20cps) 40mg;
[0041] PVP (K90) 8mg
[0042] Carbopol (934P) 16mg
Embodiment 2
[0044] Acyclovir: 600mg;
[0045] Amberlite IRP69: 500mg
[0046] Hypromellose K4M 60mg
[0047] PEG4000: 250mg;
[0048] PEG400: 7mg;
[0049] Diethyl phthalate: 6mg;
[0050] Eudragit RS100 40mg;
[0051] Eudragit RL100 30mg;
[0052] PVP (K30) 20mg
[0053] Avicel (RC591) 20mg
Embodiment 3
[0055] Acyclovir: 600mg;
[0056] Amberlite IRP69: 750mg;
[0057] Hypromellose K100M 40mg
[0058] PEG4000: 300mg;
[0059] PEG400: 10mg;
[0060] Dibutyl sebacate: 7mg;
[0061] EC (45cps) 40mg;
[0062] Tragacanth Gum 7.5mg
[0063] Carbopol (934P) 10mg
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com