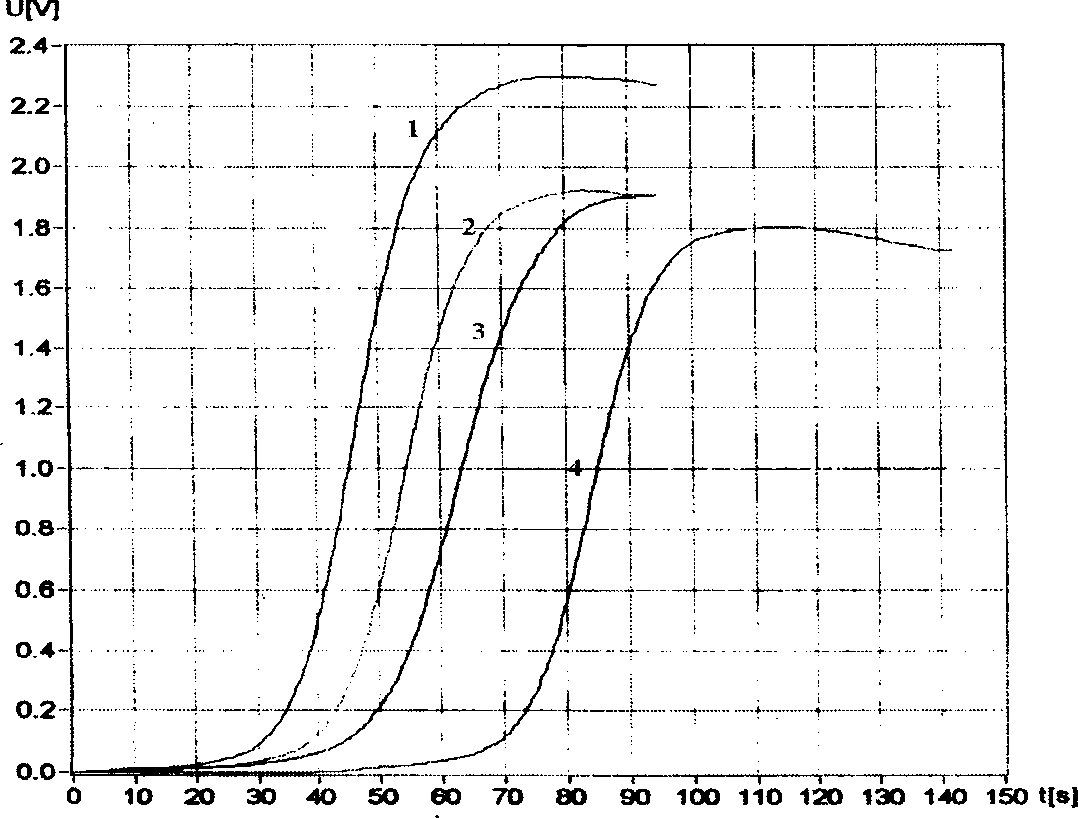
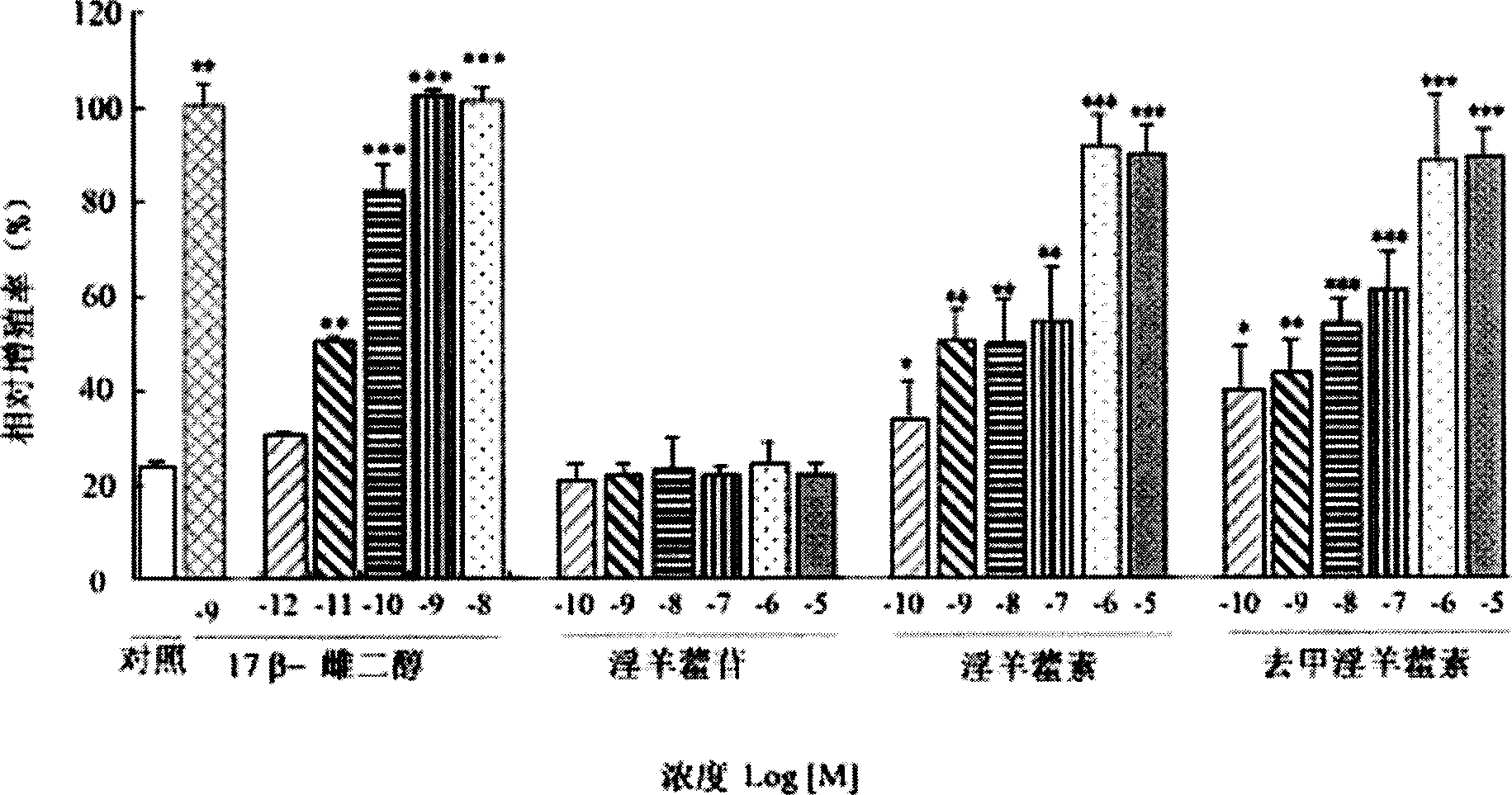
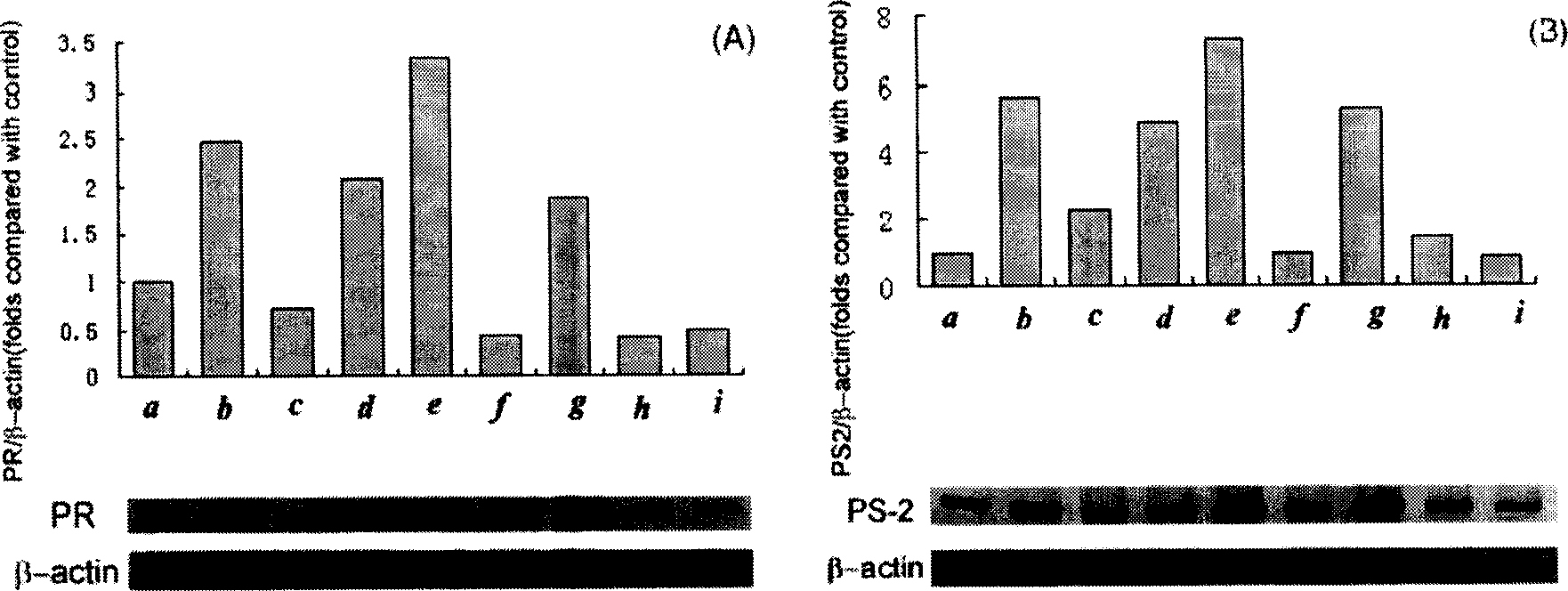
Combination of medication of containing general saponin of notoginseng and icariin as well as usage
A technology of Panax notoginseng saponins and icariin, which is applied to the composition of Panax notoginseng saponins and icariin to prepare drugs for the treatment of senile dementia, which can solve the problems of lack of adverse reactions and achieve significant anti-aging Oxidative effect, significant estrogen-like effect, effect of expanding drug use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 The preparation method of Panax notoginseng saponins and icariin of the present invention
[0021] (1) Weigh Panax notoginseng powder (20-40 mesh), add 10 times the amount of 70% ethanol to reflux and extract 3 times, each time for 2 hours, filter, combine the filtrates, and concentrate under reduced pressure to a certain volume (0.33g crude drug / ml) , that is, the column solution. Pipette the solution onto the macroporous resin, elute with 7 times the amount of 75% ethanol, and collect the eluate in sections. The eluent was evaporated to dryness in a water bath, concentrated under reduced pressure, dried in vacuo, weighed the total solids and determined the content of total saponins of Panax notoginseng. The total saponin content of this batch of Panax notoginseng > 80%.
[0022] (2) Weighing the dry raw material of Epimedium (ground part crushed material, including stems and leaves) and extracting with ethanol for 3 times (1 hour / time), concentrating and d...
Embodiment 2
[0024] Embodiment 2 The maximum tolerated dose experiment of the composition of the present invention
[0025] (1) Materials and methods:
[0026] The extracts of Panax notoginseng saponins and icariin were obtained by referring to the method in Example 1, and prepared into a suspension with 0.5% sodium carboxymethylcellulose. The drug concentration is equivalent to the content / ml in 10g of crude drug, and before use, it is mixed according to the weight ratio of Panax notoginseng saponins: icariin 0.01:0.99 to form composition A and Panax notoginseng saponins: icariin weight ratio 0.5 : 0.5 and mixed to form composition B.
[0027] Use the maximum concentration (equivalent to 10g of crude drug / ml) and the maximum administration volume of 0.4ml / 10g / time gavage administration, give the mouse composition and the single extract of the two medicinal materials twice a day, with an interval of 5 to 6 hours , observe the body weight and various symptoms of the mice on the day of adm...
Embodiment 3
[0032] Embodiment 3 The maximum tolerated dose experiment 2 of the composition of the present invention
[0033] (1) Materials and methods Refer to Example 2. The test drugs are compositions A to D of Panax notoginseng saponins and icariin in weight ratios of 0.1:0.9, 0.15:0.85, 0.2:0.8, and 0.4:0.6.
[0034] (2) Results:
[0035] The mice had loose stools on the day and the next day of intragastric administration, and dilated auricular vessels on the day of administration, but there was no significant difference among the compositions A to D. No death occurred in all the administration groups, and no apparent adverse symptoms were observed in the subsequent two weeks.
[0036] (3) Conclusion:
[0037] Compositions A to D mice were intragastrically administered with 800 g of the corresponding content / kg of the crude drug, except for loose stools and auricular vasodilation on the day of administration, no death or other obvious toxic reactions occurred.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com