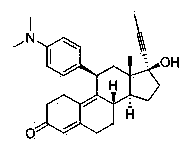
Mifepristone lipidosome preparation and preparation method thereof
A technology of mifepristone lipid and mifepristone, which is applied in the direction of liposome delivery, medical preparations of non-active ingredients, and pharmaceutical formulations, and can solve the problems of long retention time, poor solubility of mifepristone, and packaging High encapsulation rate and other issues, to achieve the effect of reducing intake, broadening clinical application, and high encapsulation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
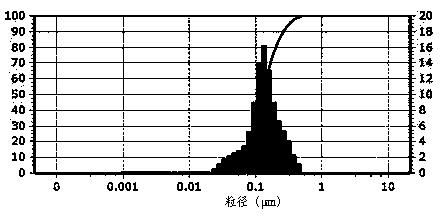
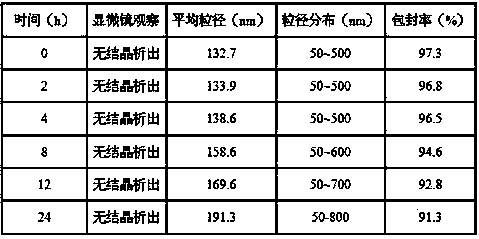
[0027] Accurately weigh 100 mg of egg yolk lecithin, 10 mg of cholesterol, 5 mg of PEG2000-DSPE, and 8 mg of mifepristone and dissolve them in 10 ml of dichloromethane. Remove the dichloromethane under pressure, and after forming a uniform transparent film on the wall of the eggplant-shaped flask, add 10 ml of 0.02 mol / L pH 7.2 phosphate buffer solution, and continue to rotate in a water bath at 37°C for 30 minutes under normal pressure to make the film swell and hydrate , and the resulting suspension was ultrasonically treated with a cell pulverizer for 5-10 min in an ice bath to obtain a mifepristone liposome solution. Add 10 ml of 0.02 mol / L pH 7.2 phosphate buffer solution for dilution, add 2.0 g of sucrose as a freeze-drying protectant, filter and sterilize with a 0.22 μm microporous membrane, and pack to obtain the finished product. The finished product can be stored at 2°C-8°C or freeze-dried to make freeze-dried powder for injection. After determination, the encapsula...
Embodiment 2
[0029] Accurately weigh 100 mg of egg yolk lecithin, 10 mg of cholesterol, and 8 mg of mifepristone and dissolve them together in 10 ml of dichloromethane. After forming a uniform transparent film on the wall of the eggplant-shaped flask, add 10 ml of 0.02mol / L pH 7.2 phosphate buffer solution, and continue to rotate in a water bath at 37°C under normal pressure for 30 minutes to make the film swell and hydrate, and the resulting suspension Under ice bath, use a cell pulverizer to sonicate for 5-10 minutes to obtain mifepristone liposome solution. Add 10 ml of 0.02 mol / L pH 7.2 phosphate buffer solution for dilution, add 2.0 g of sucrose as a freeze-drying protectant, filter and sterilize with a 0.22 μm microporous membrane, and pack to obtain the finished product. The finished product can be stored at 2°C-8°C or freeze-dried to make freeze-dried powder for injection. It was determined that the encapsulation efficiency of the liposome sample obtained according to the above st...
Embodiment 3
[0031]Accurately weigh 100 mg of egg yolk lecithin, 10 mg of cholesterol, 8 mg of polyethylene glycol derivatized phospholipid PEG2000-PE, and 15 mg of mifepristone, and dissolve them in 10 ml of dichloromethane, and place the resulting solution in a 250 ml eggplant-shaped In the flask, dichloromethane was removed under reduced pressure in a water bath at 37°C. After a uniform transparent film was formed on the wall of the eggplant-shaped flask, 10 ml of 0.02 mol / L pH 7.2 phosphate buffer solution was added, and the water bath was continued at 37°C under normal pressure. Rotate for 30 minutes to make the film swell and hydrate, and the resulting suspension is ultrasonically treated with a cell pulverizer for 5-10 minutes in an ice bath to obtain the mifepristone liposome solution. Add 10ml of 0.02mol / L pH 7.2 phosphate buffer solution for dilution, add 2.0 g of sucrose as a proppant, filter and sterilize with a 0.22 μm microporous membrane, and pack to obtain the finished produ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com