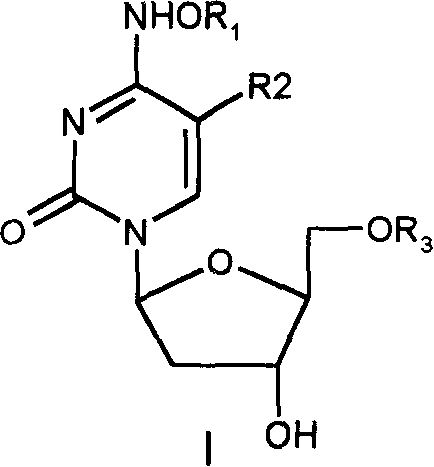
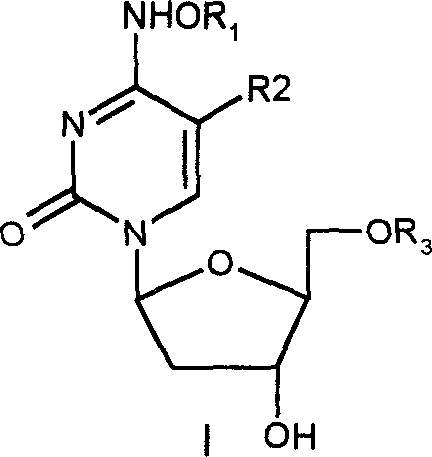
Beta-2'-deoxygenation-ramification of nucleotide, synthetic method and application of medication
A technology of derivatives and nucleosides, applied in the field of new nucleoside derivatives, can solve problems such as anti-HBV that have not been seen yet, and achieve the effects of less toxic side effects, high anti-HBV activity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
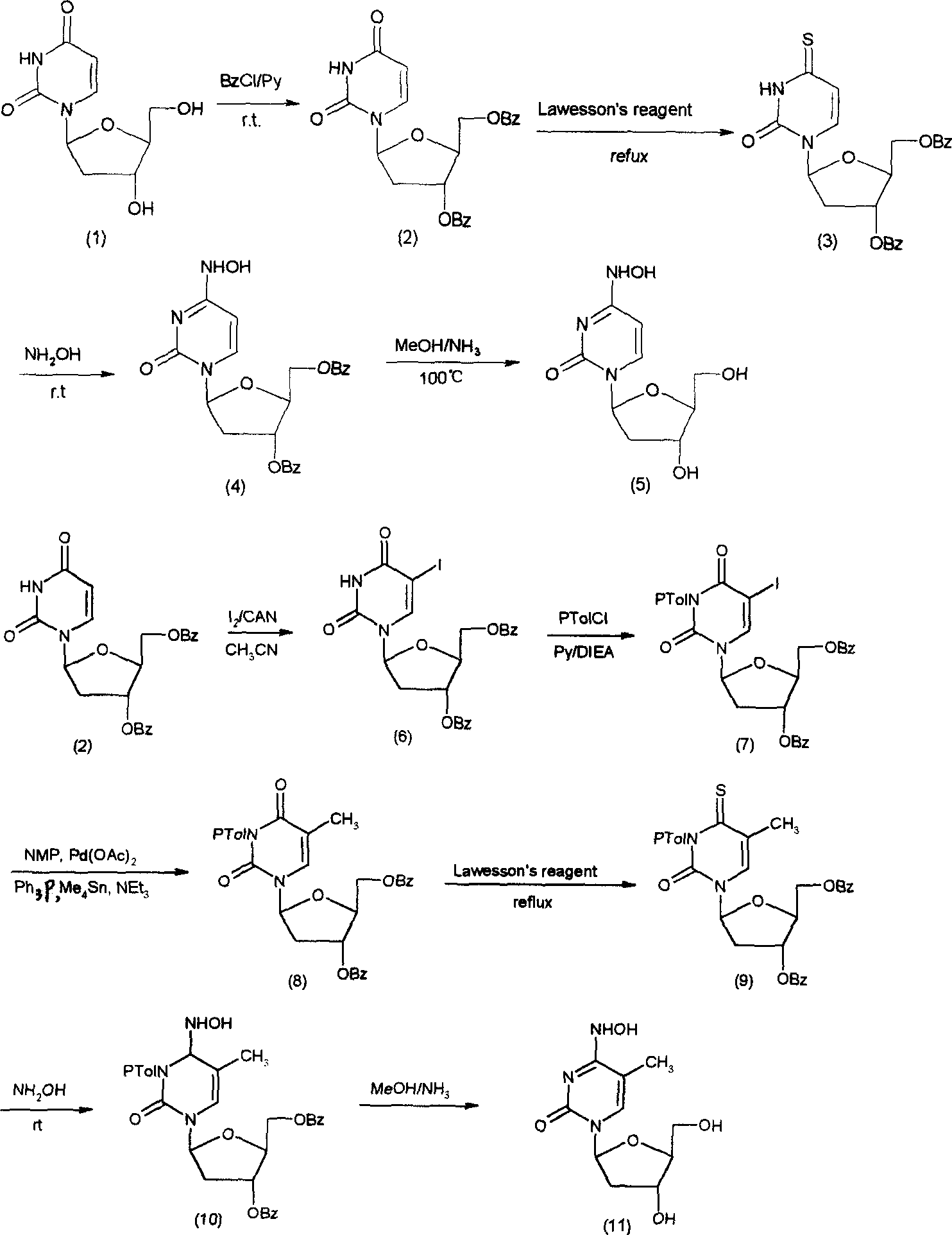
Embodiment 1
[0030] (A) Synthesis of 3',5'-dibenzoyloxy-2'-deoxy-β-L-uridine (2)
[0031] 2'-deoxy-β-L-uridine (1) (1g, 4.4mmol) was dissolved in anhydrous pyridine (20mL), and N 2 Benzoyl chloride (2 mL) was slowly added dropwise under protection. After completion, the reaction at room temperature was carried out for 6 hours. TLC detected that the starting point disappeared, and the column separation gave 1.88 g (98.4%) of compound 2, m.p.218-220°C.
[0032] 1 H NMR (CDCl 3 ): δppm 2.20-2.40 (1H, m), 2.60-2.80 (1H, m), 4.40-4.60 (3H, m), 5.62 (2H, m), 6.41 (1H, q), 7.48-8.10 (11H, m), 9.20 (1H, s, br).
[0033] (B) Synthesis of 3'5'-dibenzoyloxy-2'-deoxy-β-L-4-thio-uridine (3)
[0034] Compound 2 (220mg, 0.5mmol) and Lawsson's reagent (408mg, 1.0mmol) were put into 1,2-dichloroethane (20mL), heated to reflux for 20h, TLC detected that the raw material spot disappeared, cooled to room temperature, washed with water, concentrated and dried , column separation yielded 225 mg (98.7%)...
Embodiment 2
[0042] (A) Synthesis of 3',5'-dibenzoyloxy-2'-deoxy-5-iodo-β-L-uridine (6)
[0043] Compound 4 (1g, 2.40mmol), I 2 (0.8g), cesium ammonium nitrate (CAN) (0.7g), dissolved in acetonitrile (30mL), stirred and reacted at 85°C for 5h, TLC detected that the raw material point disappeared, and cooled to room temperature to precipitate 60.9g (69.8%) of compound, m.p. 190-192°C.
[0044] 1 H NMR (CDCl 3 )δppm: 2.31 (1H, m), 2.61-2.72 (1H, m), 4.47-4.52 (1H, m), 4.68-4.71 (2H, m), 5.58 (1H, m), 6.35 (1H, q) , 7.31-8.21 (11H, m), 9.21 (1H, s).
[0045] (B) Synthesis of 3',5'-dibenzoyloxy-2'-deoxy-3-N-toluoyl-β-L-thymidine (8)
[0046] Compound 6 (1.0g, 1.78mmol) was dissolved in anhydrous pyridine (20mL), then ethyldiisopropylammonium (DIEA) (0.7mL) was added, and p-toluoyl chloride (0.8mL ), complete and then react at room temperature for 3h, TLC detects that the raw material point disappears, add a small amount of water to stop the reaction, and use CH 2 Cl 2 Extraction, w...
Embodiment 3
[0059] (A) Synthesis of 3,'5'-dibenzoyloxy-2'-deoxy-5-fluoro-β-L-uridine (13)
[0060] Carry out the same method as Example 1, except that compound 12 is used instead of compound 1 for the reaction, and the product is determined to be 3',5'-dibenzoyloxy-2'-deoxy-5-fluoro-β-L - Uridine (13), yield 96.8%, m.p. 171-173°C.
[0061] 1 H NMR (CDCl 3 )δppm: 2.31-2.42 (1H, m), 2.59-2.80 (1H, m), 4.72-4.84 (3H, m), 5.73 (1H, m), 6.28 (1H, q), 7.35-8.08 (11H, m), 9.45 (1H, s, br).
[0062] (B) Synthesis of 3',5'-dibenzoyloxy-2'-deoxy-5-fluoro-β-L-uridine (14)
[0063] Carry out the same method as Example 1, except that compound 13 is used instead of compound 2 for the reaction, and the product is determined to be 3', 5'-dibenzoyloxy-2'-deoxy-5-fluoro-β-L- 4-Thio-uridine (14), yield 98.9%, m.p.168-169°C.
[0064] 1 H NMR (CDCl 3 )δppm: 2.33-2.44 (1H, m), 2.60-2.79 (1H, m), 4.73-4.83 (3H, m), 5.81 (1H, m), 6.32 (1H, q), 7.31-8.07 (11H, m), 9.48 (1H, s, br).
[0065] (C) Sy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com