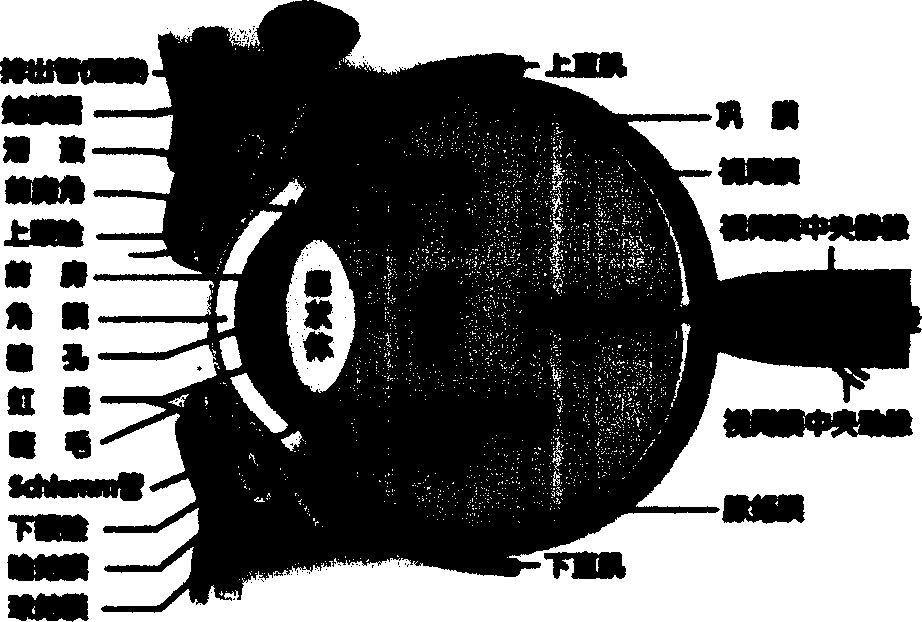
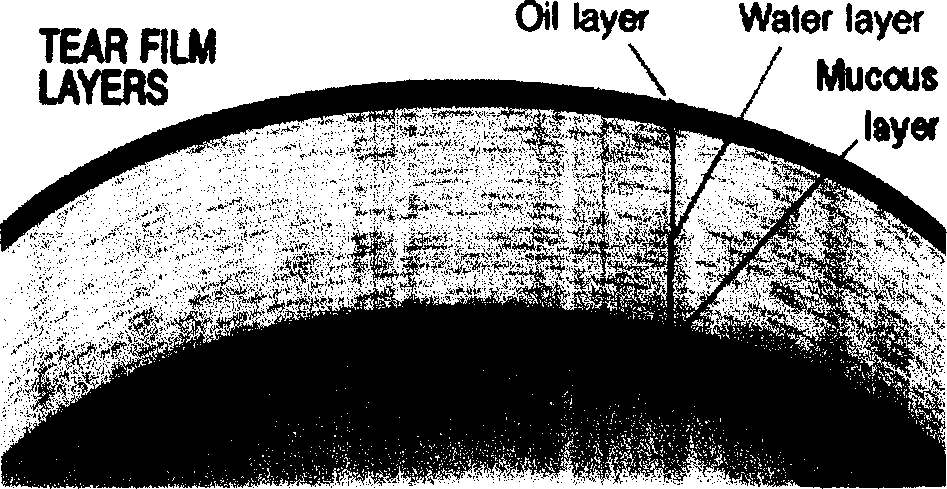
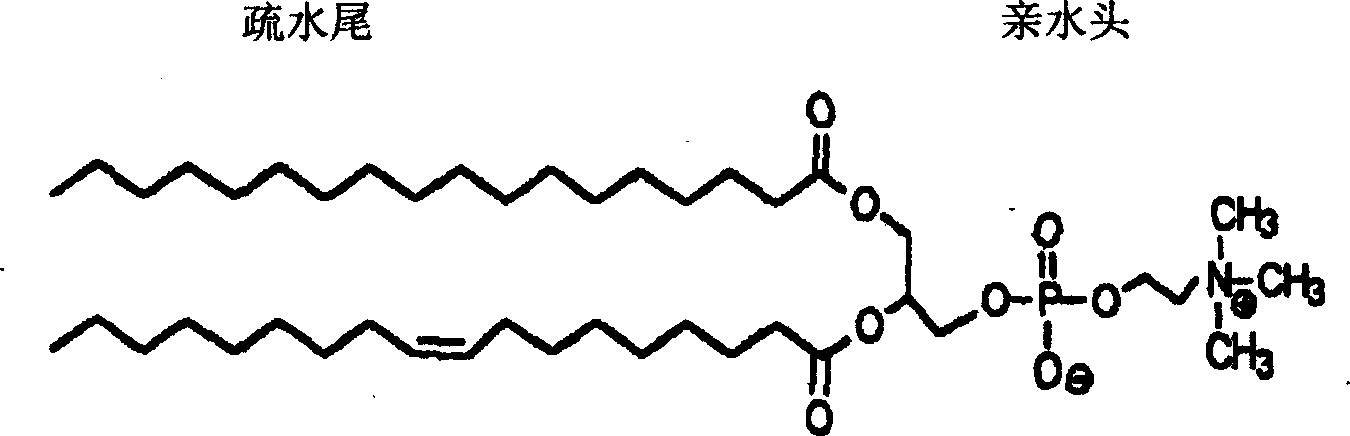
Eye formulation administering system containing lecithin and sodium hyaluronic acid and its preparing method
A technology of sodium hyaluronate and drug delivery system, which is applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, and medical preparations with non-active ingredients. Difficult to blink etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The ophthalmic drug delivery system of the present invention comprises 0.1 gram of lecithin, 0.1 gram of sodium hyaluronate, 0.85 gram of sodium chloride, 0.05 gram of borax, 0.05 gram of vitamin E acetate, and the rest is purified water in 100 mL.
Embodiment 2
[0045] The ophthalmic drug delivery system of the present invention, in 100mL, contains 0.1 gram of lecithin, 0.1 gram of sodium hyaluronate, 0.75 gram of sodium chloride, 0.3 gram of propylene glycol, 800.3 gram of Tween, 0.05 gram of borax, 0.05 gram of vitamin E acetate, and the rest are purified water.
Embodiment 3
[0047] The ophthalmic drug delivery system of the present invention, in terms of 100 mL, contains 0.1 g of lecithin, 0.1 g of sodium hyaluronate, 0.75 g of sodium chloride, 0.3 g of propylene glycol, 800.3 g of Tween, 0.05 g of borax, 0.05 g of vitamin E acetate, and hydroxybenzene 0.03 g of ethyl ester, and the rest is purified water.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com