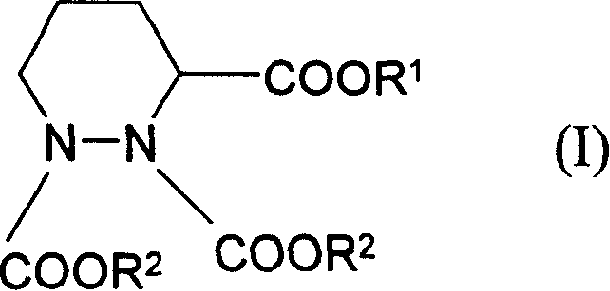
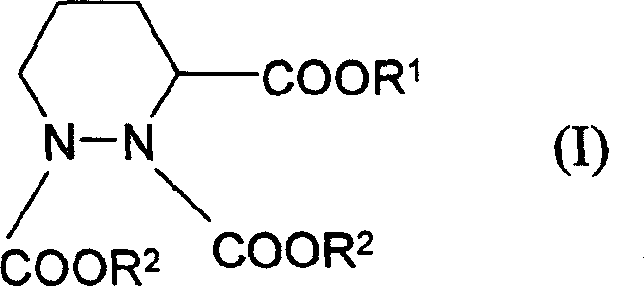
Process for preparing hexahydro pyridazine tricarboxylate
A technology of pyridazine tricarboxylate and sodium hydride, applied in directions such as organic chemistry, can solve problems such as expensive reagents and low product yield, and achieve the effects of improving reaction yield and product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0025] The present invention will be described in more detail below in conjunction with examples.
[0026] Raw material example
[0027] Preparation of ethyl 2,5-dibromopentanoate
[0028] Bromine (64.57 g, 99% purity, 0.40 mol) was added to delta-valerolactone (41.72 g, 96% purity, 0.40 mol) and PBr at a temperature of 110-115°C over 2 hours. 3 (2.20g, 0.008mol) below the liquid level of the mixed solution, wherein the second half needs to be added more slowly. After 60 minutes, the solution was cooled to 0-5°C and cold ethanol containing 3.65 g (0.10 mol) of dry HCl gas was added. After stirring at room temperature for 22 hours, ethanol and water were removed by distillation under reduced pressure (toluene or other suitable solvents can also be used to remove the water-ethanol azeotrope). The crude product was extracted with ether (200ml) and washed with saturated aqueous sodium bicarbonate (total 98ml, 0.124mol) to remove any unreacted acid. After removing the solvent b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com