Isothiazolone composition and method for stabilizing isothiazolone
A technology of isothiazolinone and composition, applied in the directions of botanical equipment and method, animal repellent, plant growth regulator, etc., can solve the problem of not using and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
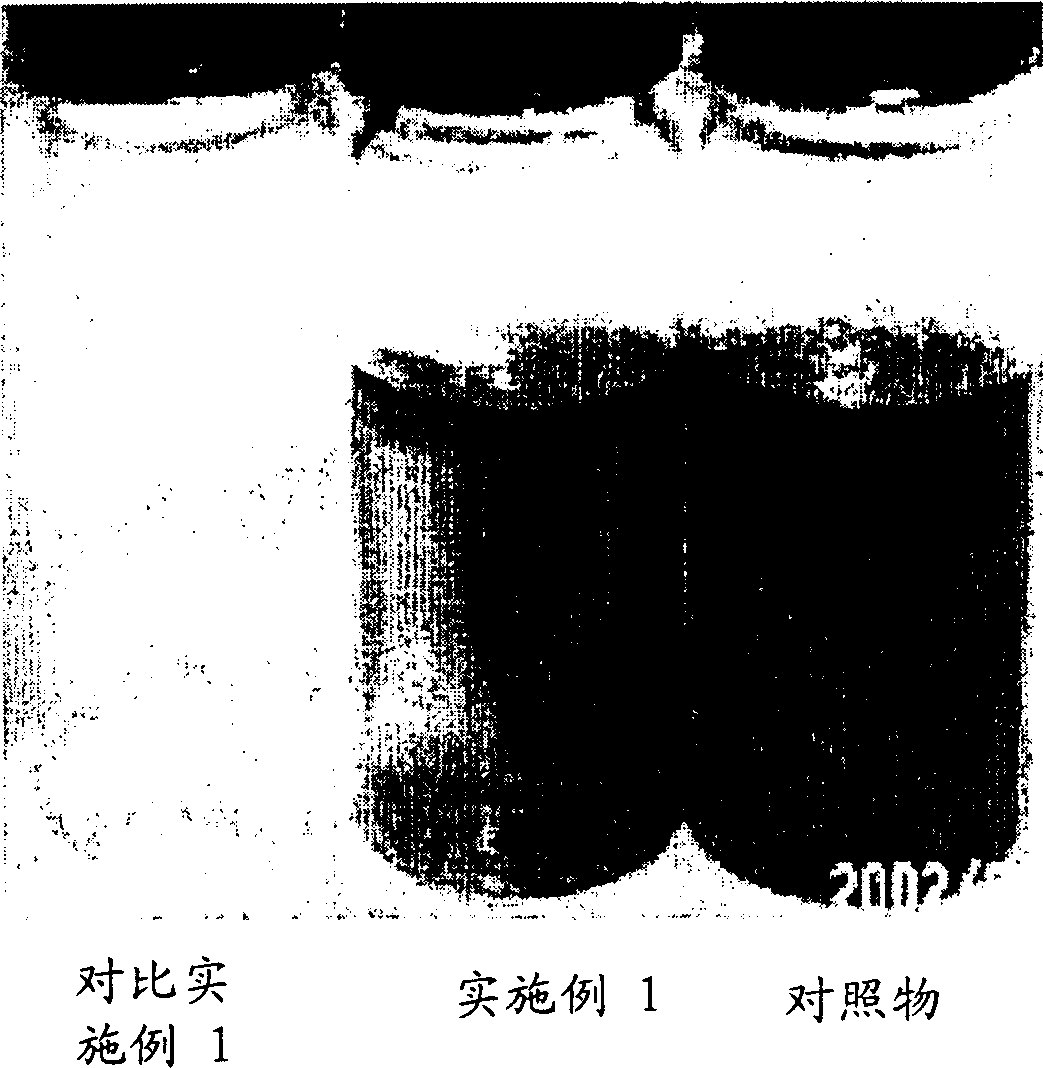
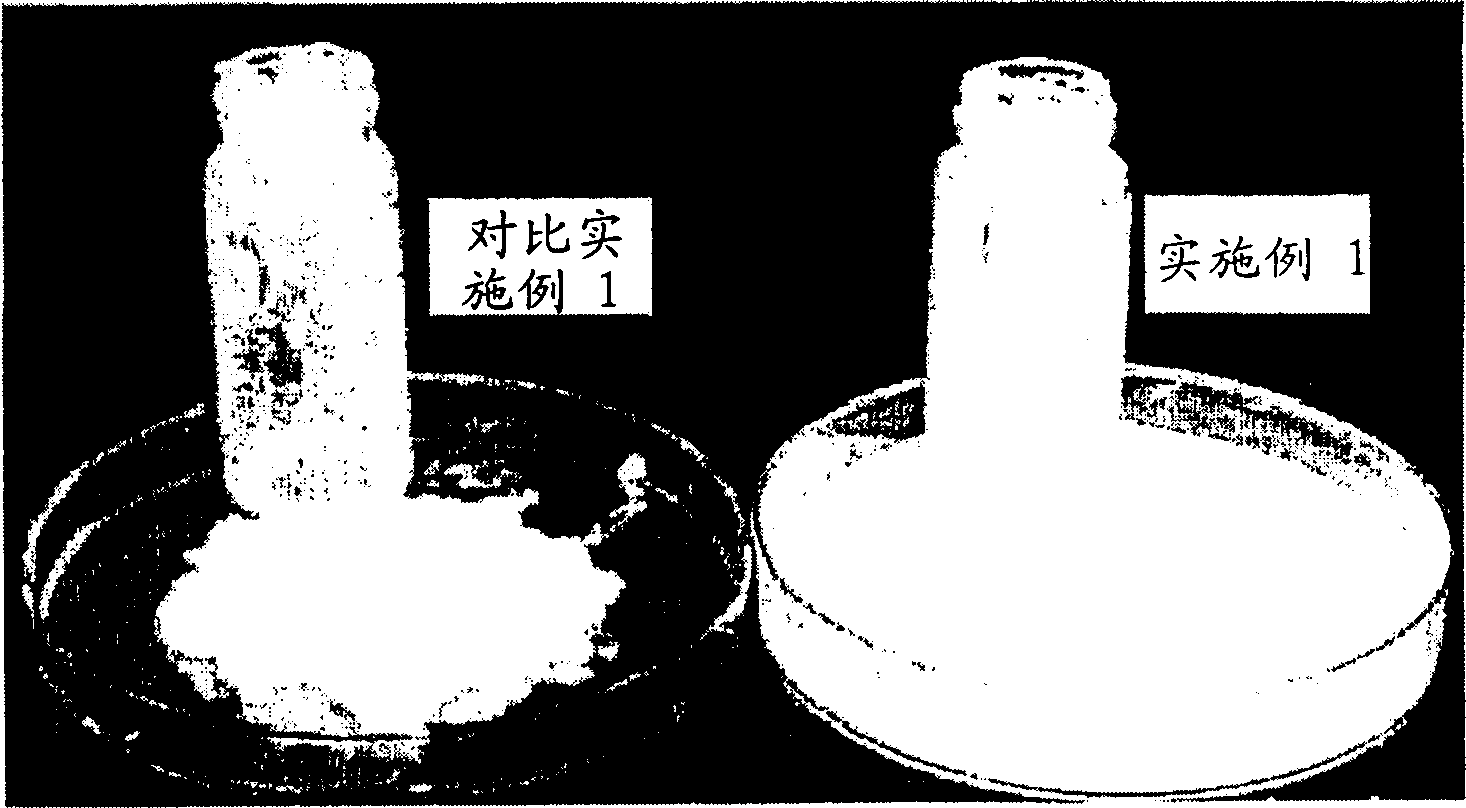
Embodiment 1-13 and comparative Embodiment 1
[0083] The concentrated solution of 3-isothiazolinone was prepared, and its composition and content are shown in Table 1. As the 3-isothiazolinone compound, use 5-chloro-2-methyl-4-isothiazolin-3-one (CMI) and 2-methyl-4-isothiazoline in a mixing ratio of about 3:1 - Mixture of 3-ketones (MI).
[0084] As shown in Table 1, the samples of Examples 1-13 and Comparative Example 1 were prepared by the following method: 3-isothiazolinone compound, sodium nitrate, sodium carbonate, magnesium nitrate (comparative example), oxidation Magnesium (comparative example) was mixed with water and KIO was added to prevent precipitation 3 , NaBrO 3 and NaClO 2 .
[0085] sample
compound
Mg(NO 3 ) 2
MgCl 2
NaNO 3
NaCl
KIO 3
NaBrO 3
NaClO 2
Example 1
13.84
-
-
17.0
5.7
0.00
2
Example 2
13.84
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com