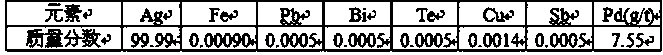Method for high-efficiency separation of palladium from silver electrolyte
An electrolyte and silver electrolytic cell technology, which is applied to the improvement of process efficiency, photography technology, instruments, etc., can solve the problems of inapplicability, impurity removal and recovery of palladium and silver electrolyte properties and composition changes, and improve production efficiency. and productivity, prolong the continuous use cycle, improve the effect of working environment conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
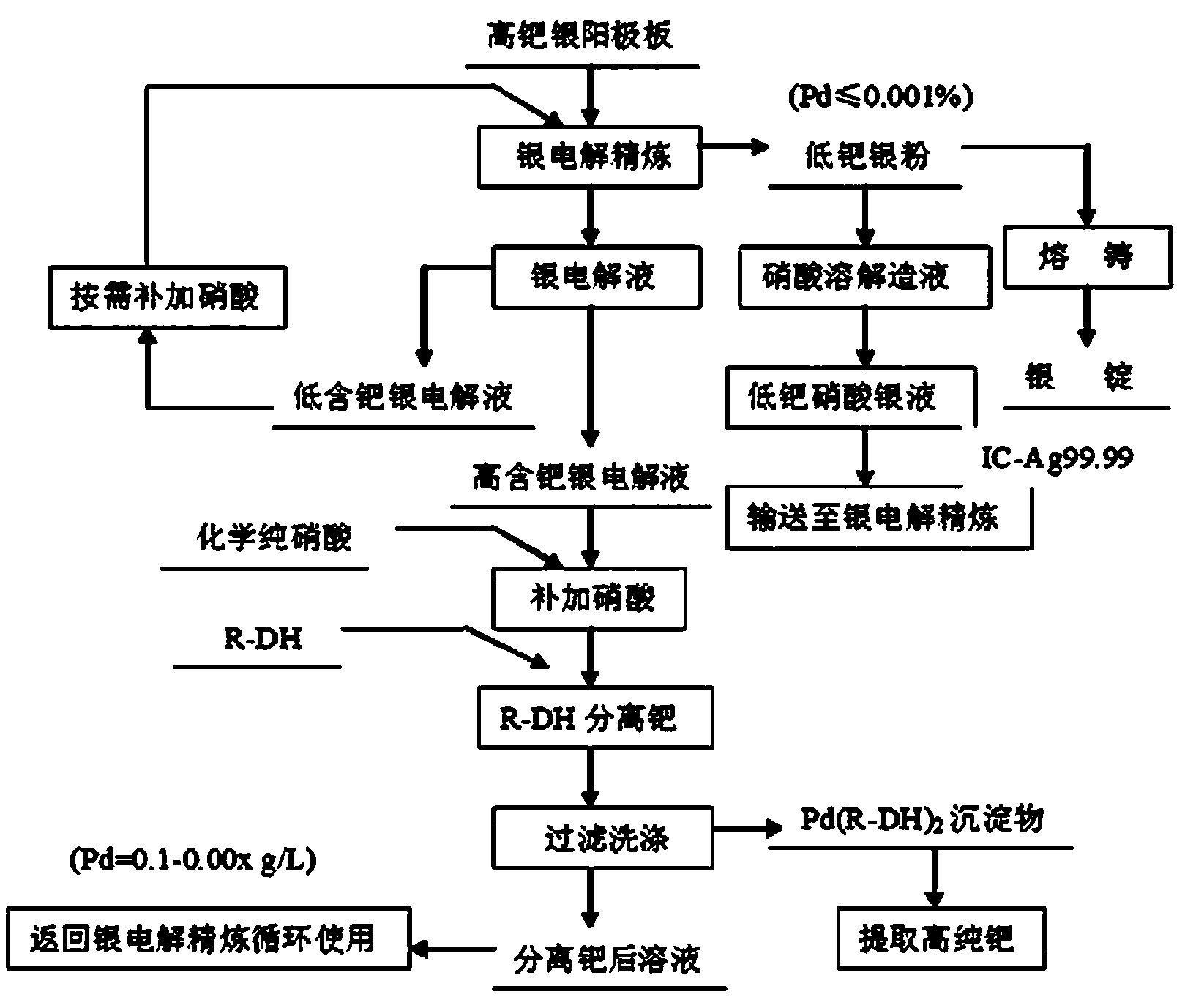
Image
Examples
Embodiment 1
[0043] Get the silver electrolyte 2000mL that element content is shown in table 1, wherein the mass volume concentration C of palladium Pd is 0.44g / L, then the quality of palladium in this silver electrolyte is 0.88g; The mass volume concentration C of free nitric acid in this silver electrolyte HNO3 Meet the requirements of 10-20g / L, heat to 35°C to obtain the heated solution; accurately weigh 1.76g high-efficiency palladium-removing agent R-DH with an electronic balance; add the weighed high-efficiency palladium-removing agent R-DH to heating The final solution was stirred and reacted for 60 minutes, and vacuum filtered to obtain the palladium-removed liquid and palladium complex. The palladium-removed liquid volume was 1950mL, and the palladium content was 0.013g / L; The separation rate was 96.8%; the quality of the palladium complex was 2.65g, and the content of each element in the palladium complex was shown in Table 2; the palladium complex was washed 2 to 3 times with ho...
Embodiment 2
[0051] Get the silver electrolyte 2000mL that element content is shown in table 5, wherein the mass volume concentration C of palladium Pd is 0.55g / L, then the quality of palladium in this silver electrolyte is 1.10g; The mass volume concentration C of free nitric acid in this silver electrolyte HNO3 Meet the requirements of 10-20g / L, heat to 40°C to obtain the heated solution; accurately weigh 2.42g high-efficiency palladium-removing agent R-DH with an electronic balance; add the weighed high-efficiency palladium-removing agent R-DH to heating The final solution was stirred and reacted for 30 minutes, and vacuum filtered to obtain the palladium-removed liquid and palladium complex. The palladium-removed liquid volume was 2000mL, and the palladium content was 0.0030g / L; it can be seen that after palladium-containing silver electrolyte, the palladium The separation rate was 98.45%; the mass of the palladium complex was 3.5 g, and the content of each element in the palladium com...
Embodiment 3
[0061] Take a silver electrolyte with a palladium mass concentration of 0.2-0.5g / L, and use an inductively coupled plasma emission spectrometer (ICP-AES) to accurately analyze and measure the mass volume concentration C of palladium in the silver electrolyte. Pd and the mass volume concentration C of free nitric acid HNO3 ; The mass volume concentration C of free nitric acid in the silver electrolyte HNO3 If it does not meet the requirement of 10-20g / L, add nitric acid to the silver electrolyte so that the mass volume concentration of free nitric acid in the silver electrolyte meets the requirement of 10-20g / L, and heat it to 45°C to obtain the heated solution Accurately weigh high-efficiency palladium-removing agent R-DH with electronic balance, the weighing M of this efficient palladium-removing agent R-DH is 1.9 times of the contained palladium quality in the silver electrolyte of getting, and the content of palladium in the silver electrolyte of getting The palladium-cont...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com