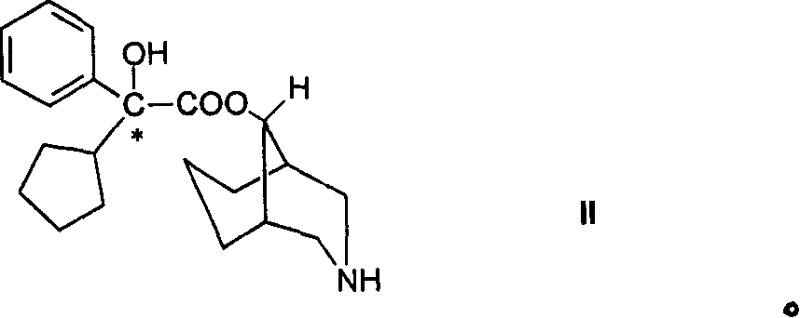
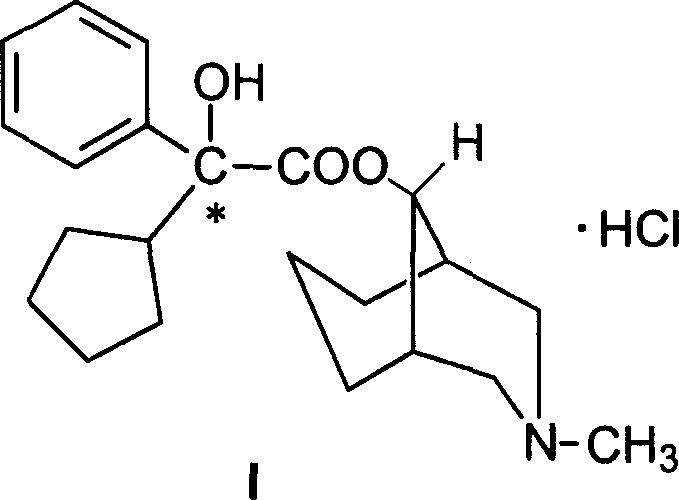
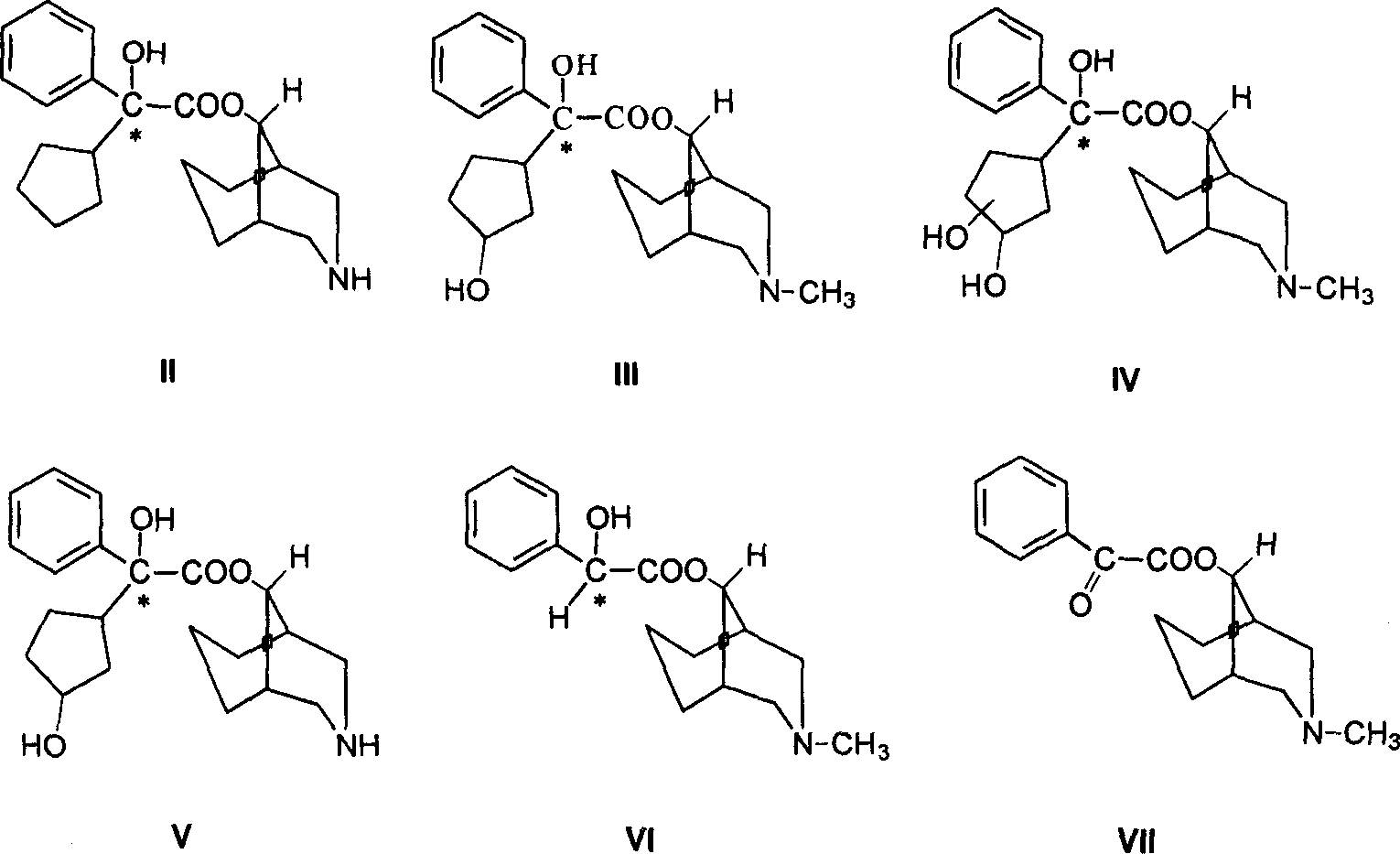
Active metabolic product of phencynonate and its medicinal use
A technology of toluenenonate and toluenenonate, which is applied in the field of active metabolites of phencyclonate and its medical application, and can solve the problem of hyperfunction of the cholinergic system, unclear pathogenesis, and dopaminergic system Poor functionality, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0033] Preparation Example 1. Preparation of Ethyl Cyclopentylglyoxylate (VIII)
[0034] Place dried 117g (0.80mol) diethyl oxalate and 120ml of anhydrous ether in a 1L three-necked flask, cool in an ice bath, protect with dry nitrogen, and add dropwise newly prepared cyclopentylmagnesium bromide Grignard reagent (0.40 mol) of anhydrous ether solution 350ml, the reaction temperature does not exceed 0°C, and the dropwise is completed within about 2.5 hours. Stir at room temperature for 2 hours, add dropwise 120 mL of an aqueous solution containing 21 g of ammonium chloride to the reaction mixture under cooling, stir at room temperature for 20 minutes, separate the ether layer, wash with water, dry over anhydrous magnesium sulfate, filter, recover the solvent, and distill the residue under reduced pressure , and collected fractions at 122-124° C. / 15 mmHg to obtain 37.3 g of the title compound (VIII) as a colorless oil, with a yield of 53%. Mass Spectrum m / z: 170(M + )
preparation example 2
[0035] Preparation example 2. Preparation of α-phenyl-α-cyclopentyl-α-glycolic acid ethyl ester (IX) and α-phenyl-α-cyclopentyl-α-glycolic acid (X)
[0036]Put 41g (0.24mol) of ethyl cyclopentylglyoxylate (VIII) and 350ml of anhydrous ether in a 1L flask, cool in an ice bath, protect with dry nitrogen, and add dropwise the newly prepared phenylmagnesium bromide Grignard reagent (0.27mol) in anhydrous ether solution (300ml), the reaction temperature does not exceed 0°C, and the dropwise is completed within about 2 hours. Stir at room temperature for 1 hour, add dropwise 100 mL of an aqueous solution containing 20 g of ammonium chloride to the reaction mixture under cooling, stir at room temperature for 20 minutes, separate the ether layer, wash with water, dry over anhydrous magnesium sulfate, filter, recover the solvent, and distill the residue under reduced pressure , collected 94-96°C / 20μmHg fractions to obtain yellow oily compound (IX), mixed with 400mL 5% methanolic potass...
preparation example 3
[0037] Preparation Example 3. Preparation of α-phenyl-α-cyclopentyl-α-glycolic acid methyl ester (XI)
[0038] Dissolve 2.2g (0.01mol) of α-phenyl-α-cyclopentyl-α-glycolic acid in 20mL of ether, and introduce diazomethane until the solution turns light yellow. The ether was evaporated to dryness. The residue was distilled under reduced pressure, and the fraction at 85-87°C / 35 mmHg was collected to obtain 2.1 g of the title compound α-phenyl-α-cyclopentyl-α-glycolic acid methyl ester (XI) as a colorless oil, with a yield of 89.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com