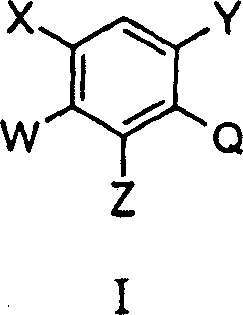
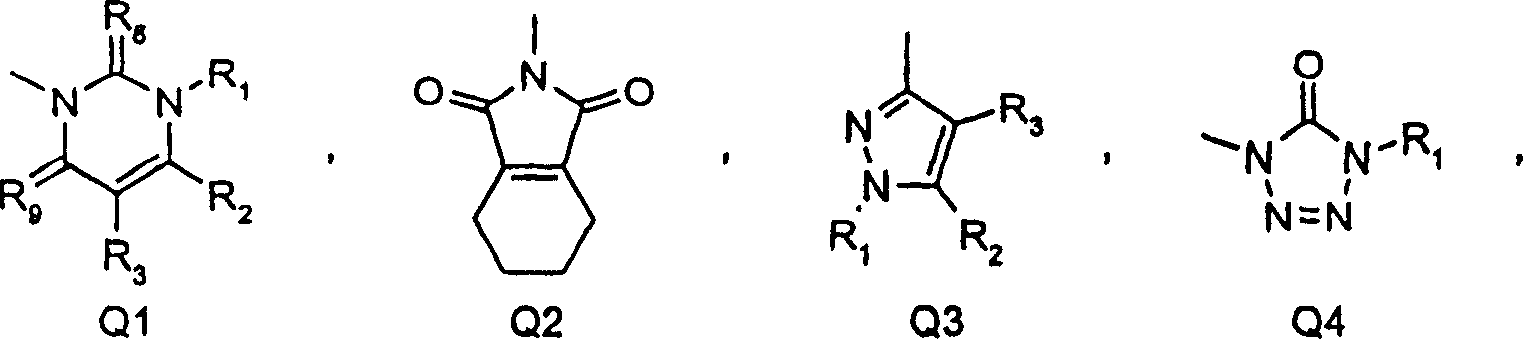
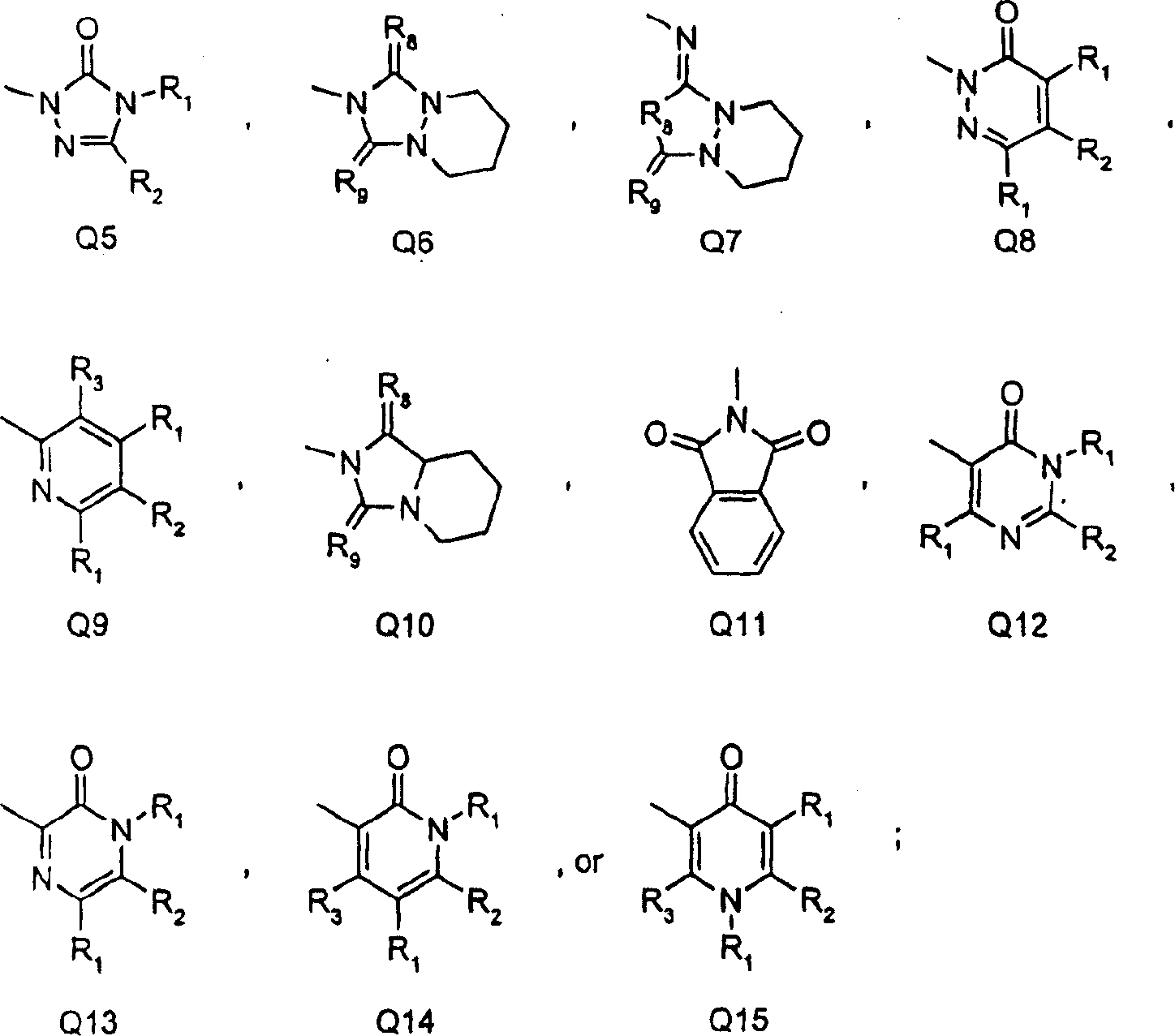
Substituted benzene compounds, process for their preparation, and herbicidal and defoliant compositions containing them
A compound, fluorophenyl technology, applied in the field of herbicide and defoliant composition, can solve the problem of not describing the general structure of the compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0078] Scheme 9 shows the preparation method of the intermediate represented by general formula V. The starting material represented by the general formula XXXIX can be prepared according to the literature method of Japanese Patent 01186849 (1989), by nitrating XXXVII to obtain XXXVIII, and then reducing to XXXIX. The amino group in XXXIX is protected by the formation of amide or carbamate XL and nitration of the latter to XLI. XLI deprotection to obtain o-nitroaniline V. V can be converted into the desired compound represented by the general formula XLV according to the method shown in the scheme.
[0079] Process 9
[0080]
[0081] (a)H 2 SO 4 -HNO 3 ; (B) Fe-AcOH; (c) pyridine-ClCOOEt (such as J=NHCOOEt); (d) H 2 SO 4 -HNO 3 ; (E) HBr-AcOH; (f) 1) triphosgene 2) NaH, 3-amino-4,4,4-trifluorobutenoic acid ethyl ester 3) CH 3 I(Q=uracil ring in X, R 1 =CH 3 , R 2 =CF 3 ); (g) ROH, alkali (example T=O, R=CH 3 )(h)Fe-AcOH(i)(CF 3 CO) 2 O (e.g. Z=NHCO CF 3 )
...
Embodiment 1
[0115] Preparation of 3-(4-chloro-6-fluoro-3-methoxy-2-nitrophenyl)-6-trifluoromethyl-2,4(1H,3H)-pyrimidinedione (Compound No. 1 -1)
[0116] At -15°C, 3-(4-chloro-6-fluoro-3-methoxyphenyl)-6-trifluoromethyl-2,4(1H,3H)-pyrimidinedione (10.0 g, 29.5mmol) was slowly added to the stirring mixture of concentrated sulfuric acid (36ml) and concentrated nitric acid (4ml). The solution was then slowly warmed to room temperature and allowed to stir for 2 hours. This solution was added to ice water to obtain a pale yellow precipitate, which was separated by filtration to obtain the title compound (9.1 g). The NMR data of this compound are listed in Table XVIII.
Embodiment 2
[0118] 3-(4-Chloro-6-fluoro-3-methoxy-2-nitrophenyl)-1-methyl-6-trifluoromethyl-2,4(1H,3H)-pyrimidinedione Preparation (Compound No. 1-5)
[0119] The 3-(4-chloro-6-fluoro-3-methoxy-2-nitrophenyl)-6-trifluoromethyl-2,4(1H,3H)-pyrimidinedione (9g, 23.5mmol ) Was dissolved in dimethylformamide (90ml), and potassium carbonate (3.9g, 28.2mmol) and dimethyl sulfate (10.2g, 47mmol) were added to this solution under stirring. The solution was stirred at room temperature for 12 hours and water was added. The product was extracted into ethyl acetate and the organic layer was washed with water and dried with anhydrous sodium sulfate. The solvent was removed to obtain the crude product, which was purified by silica gel column chromatography. The column was eluted with dichloromethane to obtain the title compound (7.8 g).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com