Novel bioactive diphenyl ethene compounds and their therapeutic applications
A compound, alkenyl technology, used in the field of pharmaceutical compositions for treating diseases and immunomodulators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
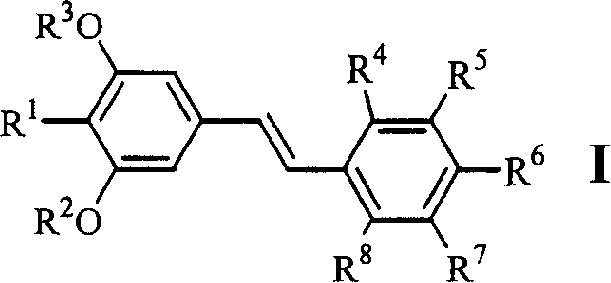
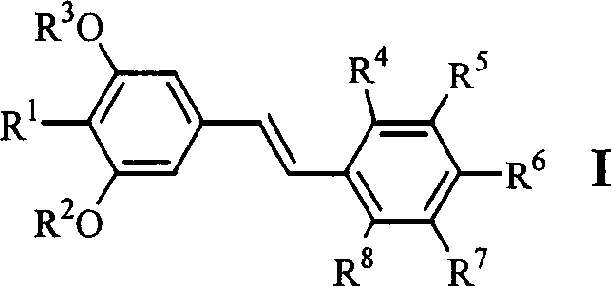
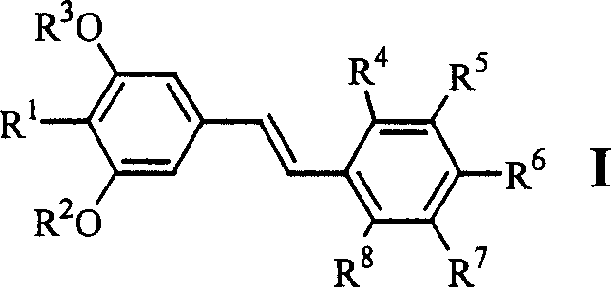
[0047] Example 1.4-[2-(3,5-dimethoxy-4-isopropylphenyl)vinyl]benzoic acid (1)
[0048] a). Methyl 3,5-dimethoxy-4-isopropylbenzoate
[0049] This compound was synthesized by the method of patent WO 01 / 42231. 1 HNMR (CDCl 3 , ppm): δ1.32(d, J=7.2Hz, 6H), 3.66(hept, J=7.2Hz, 1H), 3.82(s, 6H), 3.95(s, 3H), 7.25(s, 2H) .
[0050] b).3,5-dimethoxy-4-isopropyl benzyl alcohol
[0051] Suspend 5 g of LiAlH in 100 mL of dry diethyl ether 4 (95%, 125 mmol), under the protection of nitrogen, at 0 ℃, add dropwise 3,5-dimethoxy-4-isopropyl benzoic acid methyl ester (15.7 g, 90.1 mmol) in ether (300 mL) solution, the suspension after the dropwise addition was stirred at 0° C. for one hour, and then at room temperature for another hour. Slowly add about 10 ml of saturated Na to the reaction flask at 0°C 2 SO 4 Aqueous solution, the mixed system continued to stir overnight, the solid matter was filtered off, and the filtrate was evaporated to dryness to obtain 13.8 g of the desired pr...
Embodiment 23
[0058] Example 2.3-[2-(3,5-dimethoxy-4-isopropylphenyl)vinyl]benzoic acid (2)
[0059] This compound was synthesized from 3,5-dimethoxy-4-isopropylphenyl)ethene and 3-bromobenzoic acid by the same synthesis method as compound (1), and the yield was 77%. 1 HNMR (CDCl 3 , ppm): δ1.32(d, J=7.1Hz, 6H), 3.63(qint, J=7.1Hz, 1H), 3.90(s, 6H), 6.76(s, 6H), 7.08(d, J= 17Hz, 1H), 7.25(d, J=17Hz, 1H), 7.50(t, J=7.7Hz, 1H), 7.79(d, J=7.7Hz, 1H), 8.04(d, J=7.7Hz, 1H ), 8.31(s, 1H).
Embodiment 34
[0060] Example 3.4-[2-(3,5-dihydroxy-4-isopropylphenyl)vinyl]benzoic acid (6)
[0061]4-[2-(3,5-dimethoxy-4-isopropylphenyl)vinyl]benzoic acid (0.289 g, 0.886 mmol) and pyridine hydrochloride (0.67 g, 5.9 mmol) The mixture was heated at 200°C for 2 hours under argon flow. After the reaction mixture was lowered to room temperature, 10 ml of 2N hydrochloric acid and 50 ml of ether were added, and the organic phase and the aqueous phase were separated, and the aqueous phase was extracted twice with 50 ml of ether, and the extracts were combined, washed with saturated brine, washed with sodium sulfate dry. After the diethyl ether was distilled off, the residue was separated and purified by column chromatography, and the eluent was ethyl acetate / hexane / acetic acid (40 / 60 / 1) to obtain 4-[2-(3,5-dihydroxy-4-isopropyl phenyl) vinyl] benzoic acid (6) 0.03 g, yield 11%. 1 HNMR (DMSO-d 6 , ppm): δ1.22(d, J=7.0Hz), 6.49(s, 2H), 6.90(d, J=18Hz, 1H), 7.19(d, J=18Hz, 1H), 7.67(d, J =8Hz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com