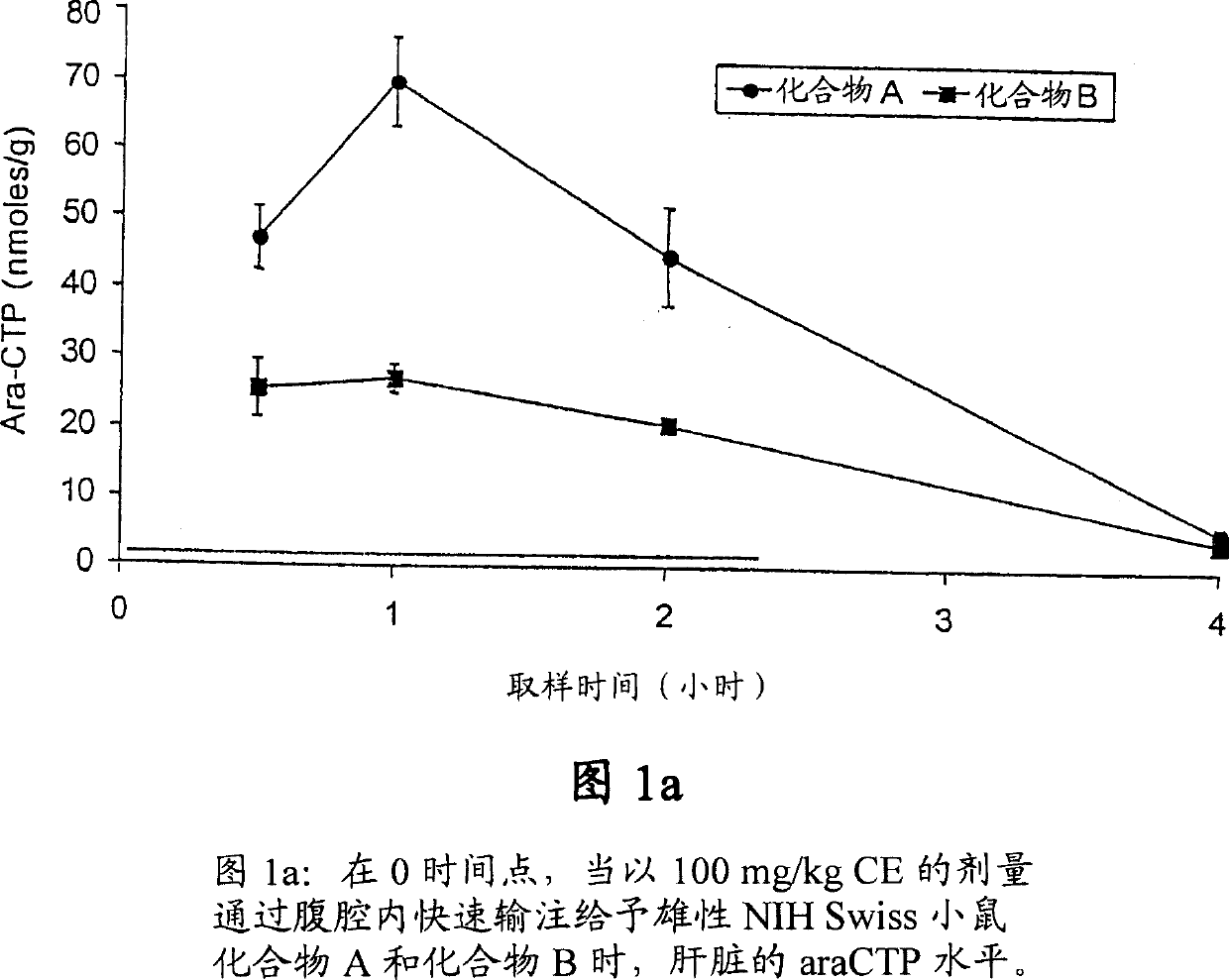
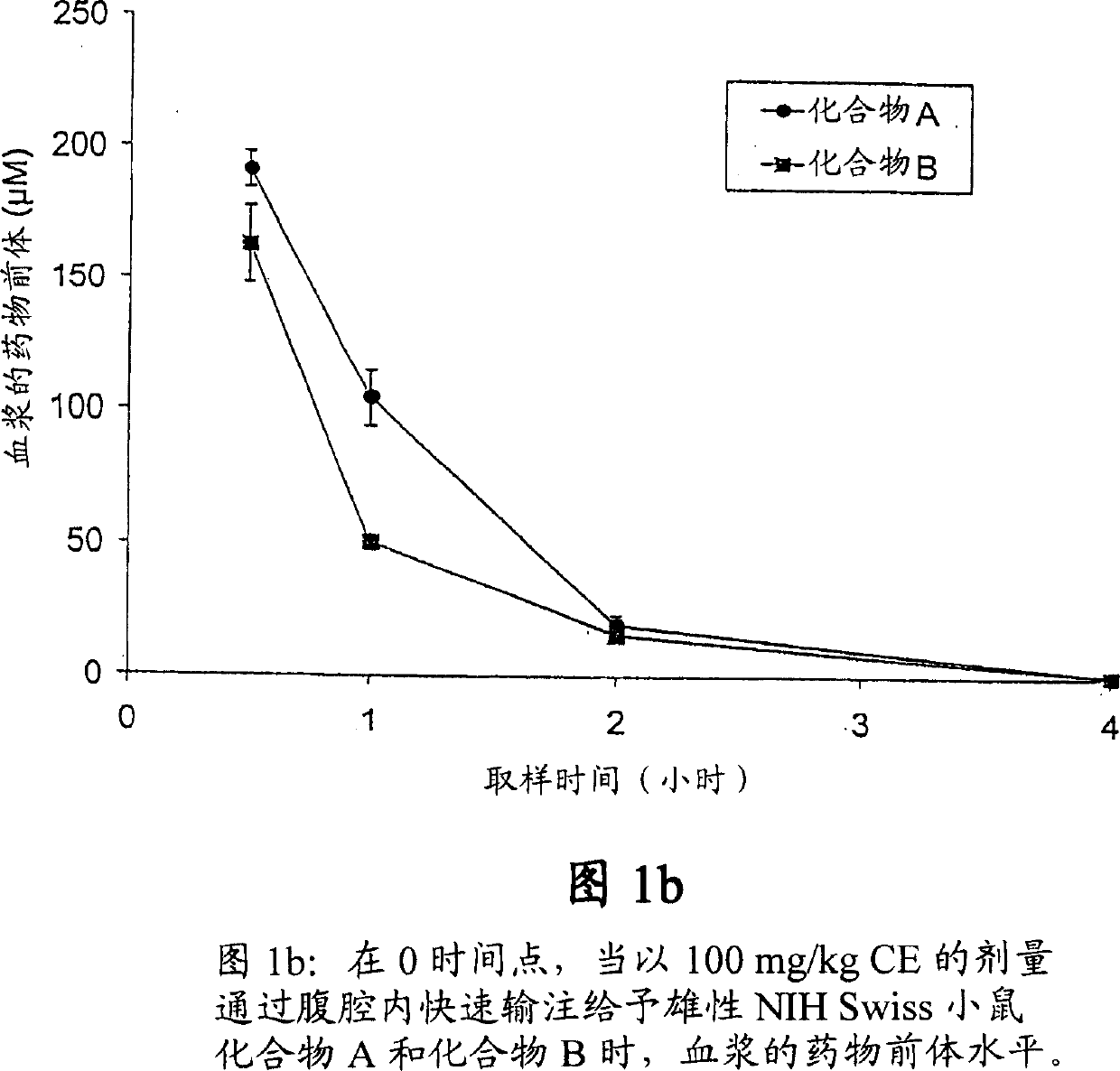
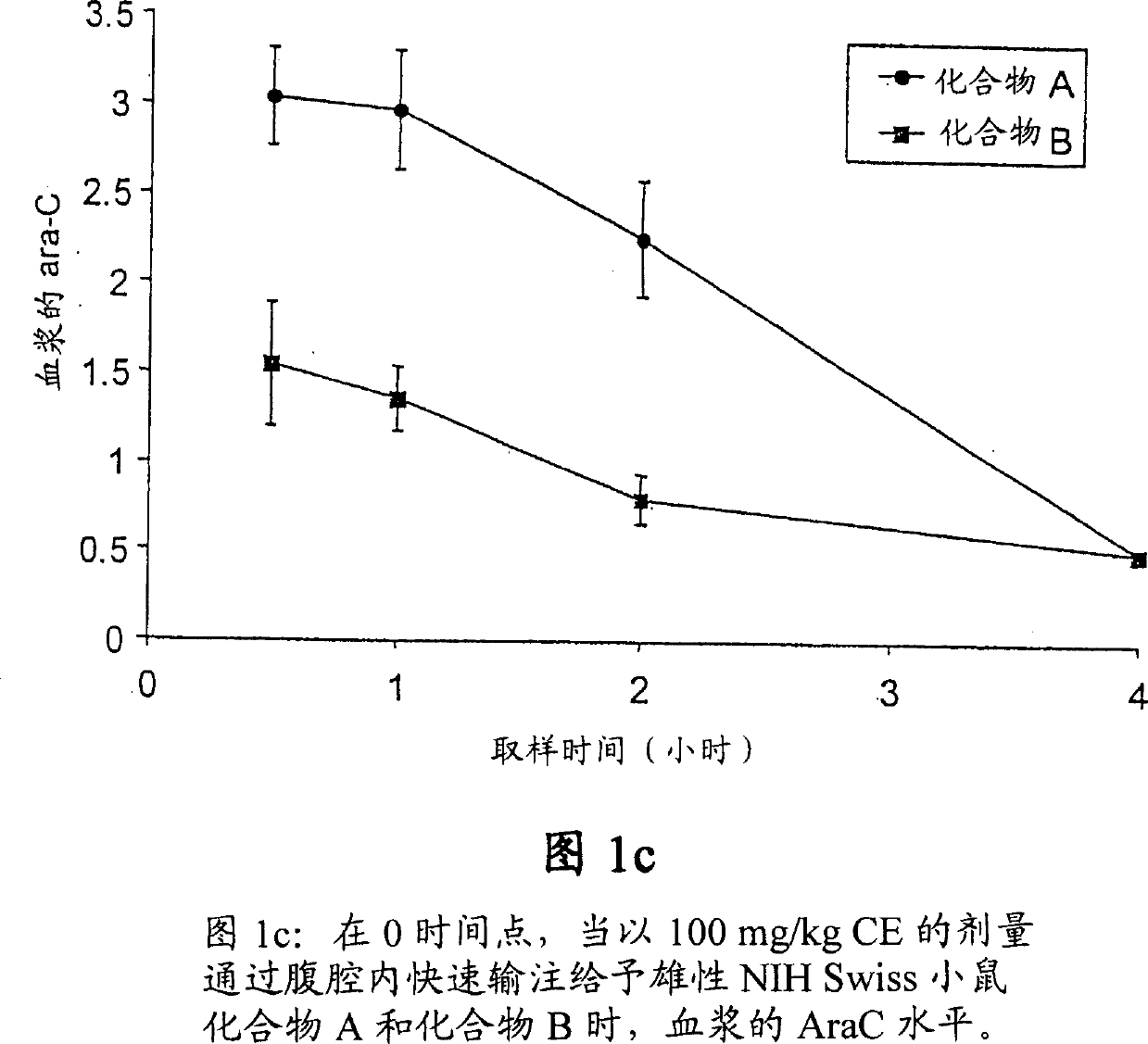
Novel cytosine monophosphate medicine precursor
A cytarabine and drug technology, applied in the field of cytarabine monophosphate cyclic diester, can solve the problems of poor efficacy of hepatitis and liver cancer, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0239] The synthesis of embodiment 1.3-oxo-3-(pyridine-4-yl)-pyruvate methyl ester (1)
[0240]
[0241] A 50 L three necked round bottom flask was equipped with an upper stirrer, heating pack and nitrogen inlet. A bottle was charged with THF (8 L) and potassium tert-butoxide (5 kg, 44.6 mol), then additional THF (18 L) was added. 4-acetylpyridine (2.5 kg, 20.6 mol) was added as the temperature increased, followed by dimethyl carbonate (3.75 L, 44.5 mol). After both ingredients were added, the temperature of the mixture was above 40°C. The reaction mixture was stirred without heating for 2.5 hours, during which time the temperature increased to an average temperature of about 55°C. The mixture was then heated to 57°C-60°C for 3 hours. The progress of the reaction was monitored by thin layer chromatography (TLC). The heat was turned off and the mixture was allowed to cool slowly overnight (15 hours). The mixture was then filtered through a 45 cm Buchner funnel. The pot...
Embodiment 2
[0244] Example 2. Synthesis of (S)-3-hydroxyl-3-(pyridine-4-yl)-acetone methyl ester (2)
[0245]
[0246] A 22 L three necked round bottom flask was fitted with upper stirrer, thermowell / thermometer, addition funnel (1 L) and cooler (empty). The flask was purged with nitrogen, charged with formic acid (877 g) and cooled with an ice bath. Triethylamine (TEA) (755 g) was charged to the addition funnel and slowly added to the stirred formic acid at intervals over 50 min. There was an exothermic reaction with moderate fuming which disappeared as the addition approached the end. When the addition was complete, the cooling bath was removed and the reaction product was diluted with dimethylformamide (DMF) (0.5 L). Ketoester 1 (2648 g) was added in one portion, followed by an additional 0.5 L of DMF in an endothermic reaction. The temperature will drop by about 5°C, which indicates that ketoester 1 is insoluble. The flask was fitted with a heating pack, and the stirred mixture...
Embodiment 3
[0252] Example 3. Synthesis of (S)(-)-1-(4-pyridine)-1,3-propanediol
[0253]
[0254]A 22 L four necked round bottom flask was fitted with upper stirrer, thermometer well / thermometer, addition funnel (2 L), condenser and cooler (empty). The flask was purged with nitrogen and charged sequentially with sodium borohydride (419 g) and 1-butanol (9.0 l). The crude hydroxyester (2) was dissolved in 1-butanol (1.0 L, the total volume of the solution was 3.2 L). A quarter (800 mL) of the hydroxyester solution was slowly added to the stirred sodium borohydride slurry at intervals over 90 minutes. The temperature increased from 19°C to 32°C and moderate gas evolution occurred. The mixture was stirred for 45 minutes, reaching a maximum temperature of 36°C. A second quarter of the hydroxyester solution was added slowly at intervals over 45 minutes, increasing the temperature from 36°C to 52°C. The mixture was stirred for 20 minutes while the maximum temperature reached 57°C. The ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com