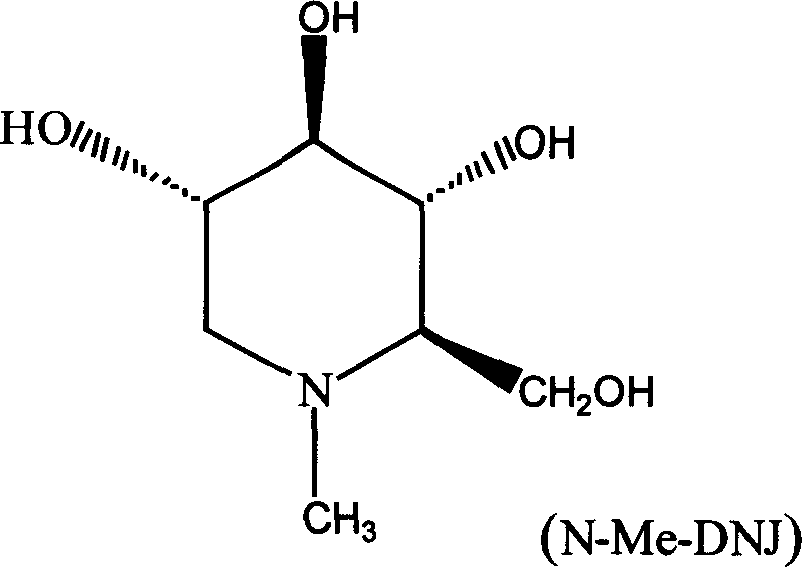
Method for extracting and preparing N-methyl-1-deoxy noijirimycin
A technology of deoxynojirimycin and methyl, which is applied in the direction of organic chemistry and can solve the problems of low yield, inability to realize industrial production, and complex process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Preparation of N-methyl-1-deoxynojirimycin from mulberry leaves.
[0017] Specific preparation process:
[0018] One kilogram of mulberry leaves, add 15 times of water, soak, heat and extract twice, each time for 2 hours, filter, concentrate to about 1 liter, add 1 liter of ethanol, and separate the supernatant with a strong acid cation exchange resin column, first Elute with 8 liters of water, then elute with 0.3M ammonia water, collect the ammonia water eluate, add 10ml each of formaldehyde and acetic acid after concentration, keep warm at 80°C for 3 hours, separate with strong acid anion exchange resin, elute with water, and Every 300ml is collected as a part, and detected by thin layer chromatography (TLC method), PrOH-AcOH-H 2 O (4:1:1) is the developer. The combined Rf value was 0.23, and the ninhydrin showed a purple-red part, which was concentrated to obtain N-methyl-1-deoxynojirimycin (1510 mg).
Embodiment 2
[0020] Extract N-methyl-1-deoxynojirimycin from Cortex Morus alba.
[0021] Specific preparation process:
[0022] One kilogram of Cortex Mori, add 10 times the amount of water, heat at 95°C to extract twice, each time for 2 hours, filter, concentrate to 1 liter, add 1 liter of ethanol, and separate the supernatant with a strong acid cation exchange resin column , first eluted with 5 liters of water, then eluted with 0.5M ammonia water, collected the ammonia water eluate, concentrated, added 15ml each of formaldehyde and formic acid, heated at 60°C for 5 hours, separated with strong acid anion exchange resin, and eluted with water. Collect every 300ml as a part, detect with thin layer chromatography (TLC method), PrOH-AcOH-H 2 O (4:1:1) is the developer. The combined Rf value was 0.23, and the ninhydrin showed a purple part, which was concentrated to obtain N-methyl-1-deoxynojirimycin (1780 mg).
Embodiment 3
[0024] N-methyl-1-deoxynojirimycin was extracted by soaking at room temperature.
[0025] Specific preparation process:
[0026] One kilogram of mulberry leaves, 15 times of water, extracted twice at room temperature, 24 hours each time, filtered, concentrated to about 1 liter, added 1 liter of methanol, and the supernatant was separated with a strong acid cation exchange resin column, and first eluted with 5 liters of water , then elute with 0.2M ammonia water, collect the ammonia water eluate, add 10ml each of formaldehyde and acetic acid after concentration, heat at 70°C for 5 hours, separate with strong acid anion exchange resin after concentration, and elute with water, with each 300m1 as a part Collect, detect with thin layer chromatography (TLC method), PrOH-AcOH-H 2 O (4:1:1) is the developer. The combined Rf value was 0.23, and the ninhydrin showed a purple-red part, which was concentrated to obtain N-methyl-1-deoxynojirimycin (950 mg).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com