Positive electrode active material for lithium secondary cell and lithium secondary cell
A cathode active material, lithium battery technology, applied in nanotechnology for materials and surface science, battery electrodes, active material electrodes, etc., can solve problems such as reduced discharge capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
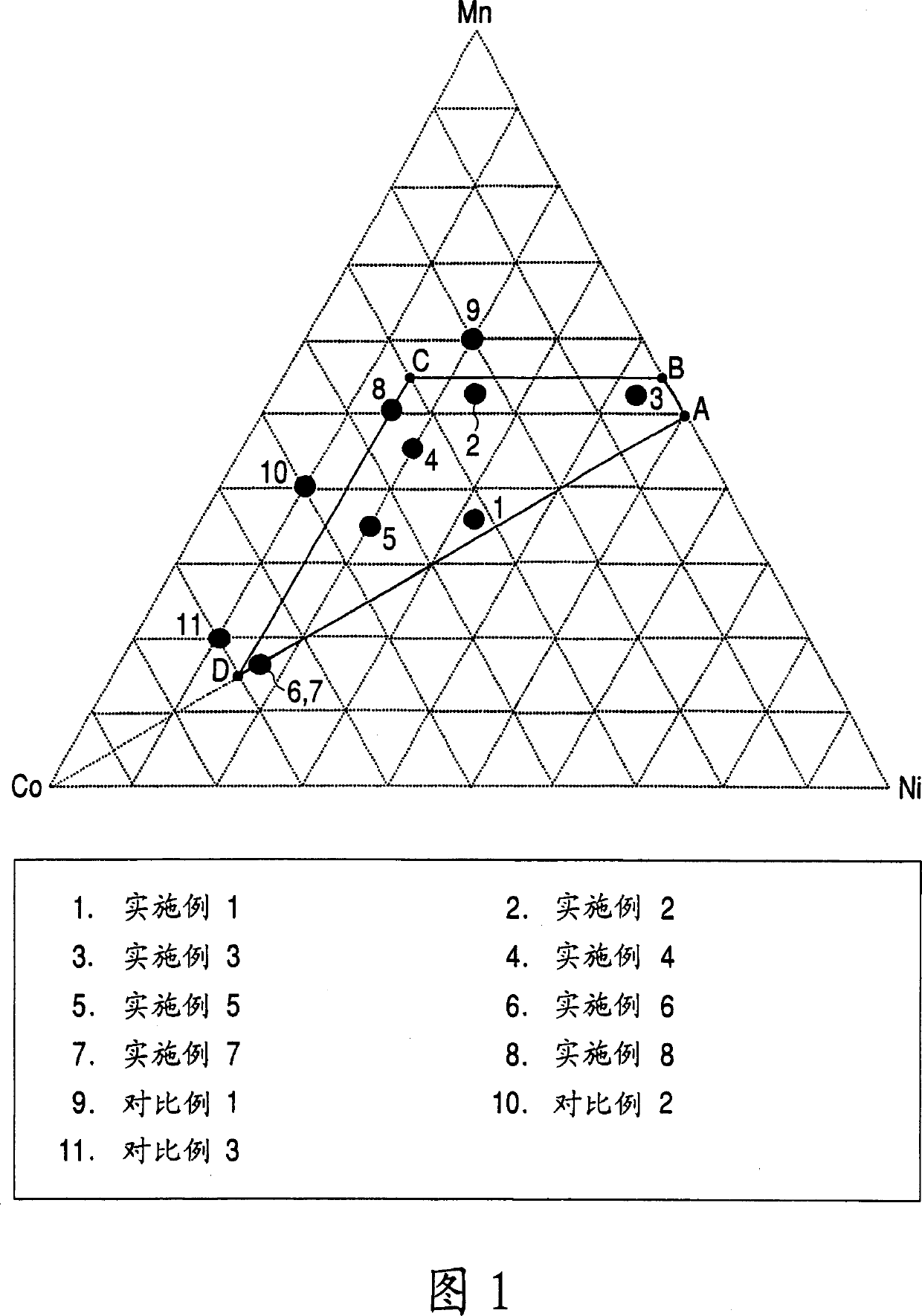
Embodiment 1
[0136] 3L (liter) of water was introduced into the closed reactor. A 32% aqueous sodium hydroxide solution was added thereto to bring the pH to 11.6. The contents were stirred at 1200 rpm with a stirrer having paddle-type stirring blades, and the temperature of the solution in the reactor was maintained at 50° C. with a heater. In addition, argon gas was passed through the solution in order to remove dissolved oxygen.
[0137] On the other hand, a stock solution is prepared. Manganese sulfate (MnSO 4 ) aqueous solution and nickel sulfate (NiSO 4 ) aqueous solution, cobalt sulfate (CoSO 4 ) aqueous solution and hydrazine (NH 2 NH 2 ) aqueous solution is mixed so as to obtain the solution that the manganese concentration is 0.633mol / L, the nickel concentration is 0.563mol / L, the cobalt concentration is 0.563mol / L and the hydrazine concentration is 0.0101mol / L, thus obtaining the raw material ready to use in the present embodiment solution.
[0138] While continuously sti...
Embodiment 2
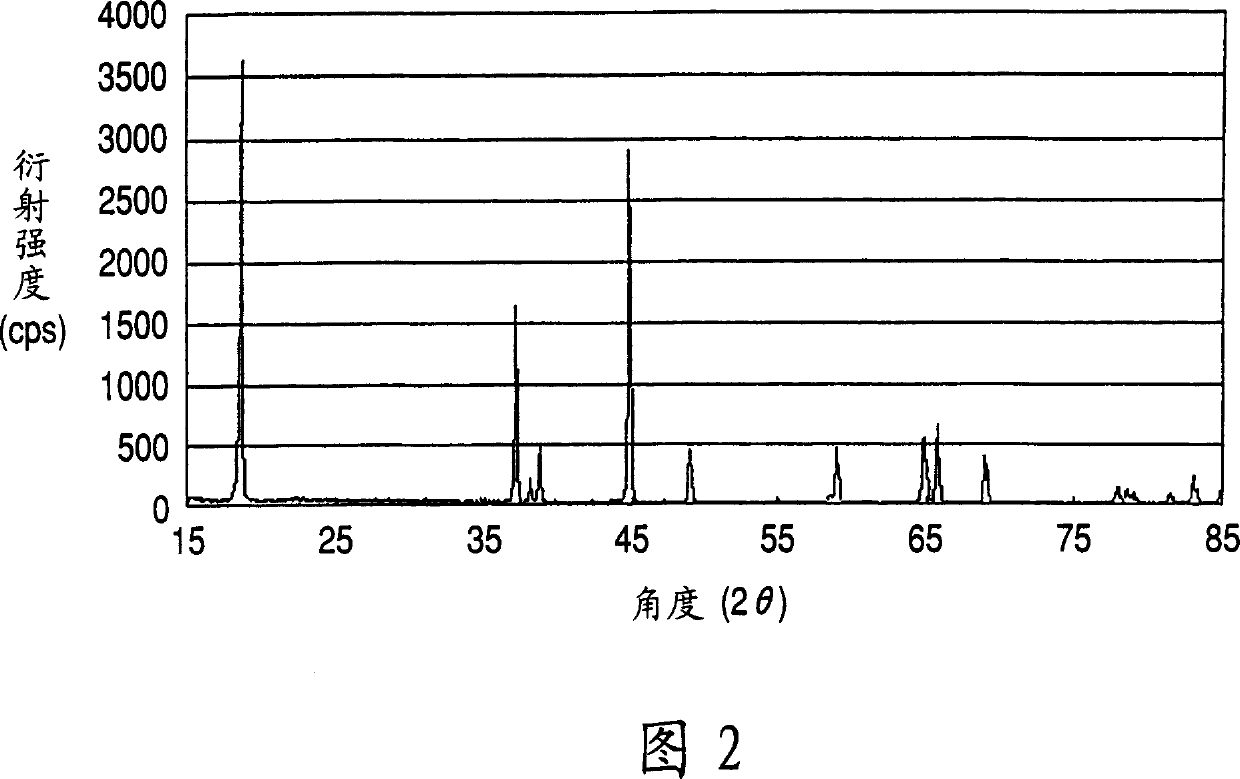
[0141] Manufacture the Mn-Ni-Co co-precipitation precursor according to the same method as in Example 1, the difference is that the raw material solution used is manganese sulfate (MnSO 4 ) aqueous solution and nickel sulfate (NiSO 4 ) aqueous solution, cobalt sulfate (CoSO 4 ) aqueous solution and hydrazine (NH 2 NH 2 ) aqueous solutions to obtain a solution having a manganese concentration of 0.915 mol / L, a nickel concentration of 0.422 mol / L, a cobalt concentration of 0.422 mol / L and a hydrazine concentration of 0.0101 mol / L. X-ray diffraction analysis reveals that the Mn-Ni-Co coprecipitation precursor mainly contains β-Ni(OH) 2 type crystal structure.
[0142] The obtained Mn-Ni-Co coprecipitated precursor and lithium hydroxide monohydrate powder were weighed so that the atomic ratio Li / (Ni+Mn+Co) was 1.3. In the same manner as in Example 1, a Li-Mn-Ni-Co composite oxide was obtained from the resulting mixture. The thus-obtained Li-Mn-Ni-Co composite oxide was analy...
Embodiment 3
[0144] Manufacture the Mn-Ni-Co co-precipitation precursor according to the same method as in Example 1, the difference is that the raw material solution used is manganese sulfate (MnSO 4 ) aqueous solution and nickel sulfate (NiSO 4 ) aqueous solution, cobalt sulfate (CoSO 4 ) aqueous solution and hydrazine (NH 2 NH 2 ) aqueous solutions to obtain a solution with a manganese concentration of 0.915 mol / L, a nickel concentration of 0.756 mol / L, a cobalt concentration of 0.088 mol / L and a hydrazine concentration of 0.0101 mol / L. X-ray diffraction analysis revealed that the Mn-Ni-Co coprecipitated precursor mainly contained β-Ni(OH) 2 type crystal structure.
[0145] The obtained Mn-Ni-Co coprecipitated precursor and lithium hydroxide monohydrate powder were weighed so that the atomic ratio Li / (Ni+Mn+Co) was 1.1. In the same manner as in Example 1, a Li-Mn-Ni-Co composite oxide was obtained from the resulting mixture. The thus-obtained Li-Mn-Ni-Co composite oxide was analyz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com