Phloroglucinol propionyl derivative and its synthesis method and uses
A technology of phloroglucinol and propionylphloroglucinol, which is applied in the field of phloroglucinol propionyl derivatives and their synthesis, and can solve the problems of unreported biological activity testing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
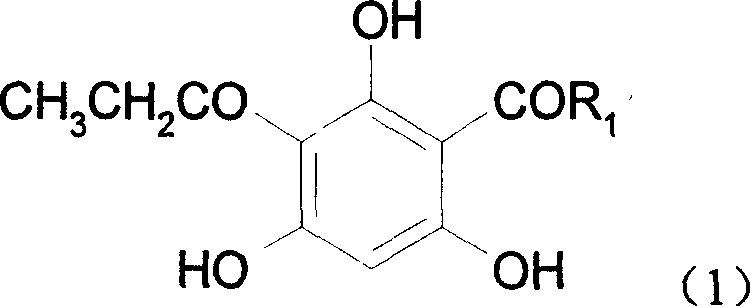
[0024] The molecular formula of 2,4-dipropionylphloroglucinol:
[0025]
[0026] Synthesis of 2,4-dipropionylphloroglucinol:
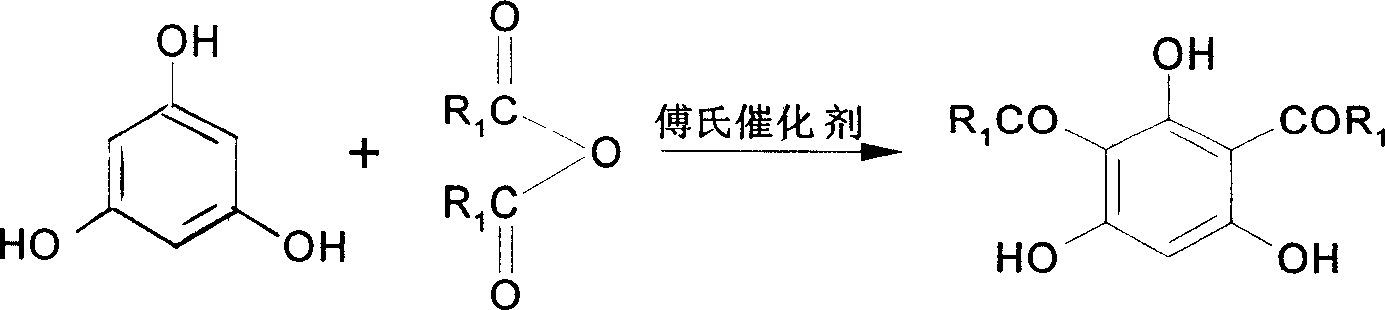
[0027] In the 250ml there-necked flask equipped with stirring device and reflux condenser, add 20g (0.16mol) phloroglucinol, at room temperature, under nitrogen protection, slowly add 42.9ml (0.33mol, 43.37g) propionic anhydride and 110ml ( 123.92 g) boron trifluoride-diethyl ether solution, wherein boron trifluoride is 0.87 mol, after the dropwise addition, keep the reaction temperature at 26-30°C and stir for 24 hours. After the reaction, add 400ml distilled water, stir for 5-10min, remove BF 3 , separated the reaction solution, the water layer was washed with 100ml ethyl acetate, the ester layer was combined with the reaction solution and the solvent was distilled off under reduced pressure, and the resulting solid was recrystallized with 60% ethanol / water to obtain the crude product, and after multiple recrystallizations, 2 , 4-dipropionylphlo...
Embodiment 2
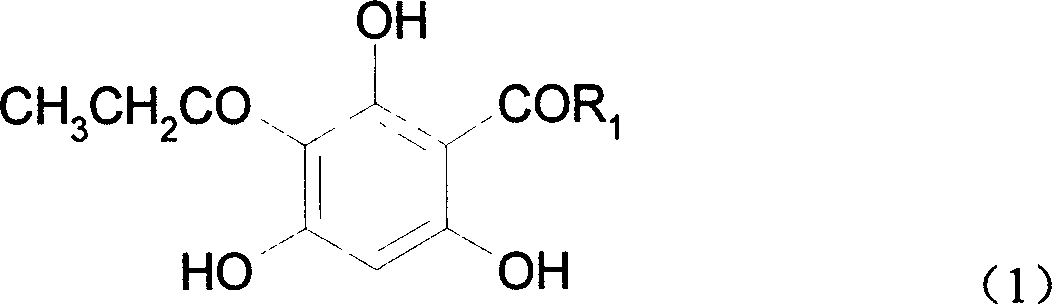
[0030] The molecular formula of 2-propionyl-4-butyrylphloroglucinol:
[0031]
[0032] Synthesis of 2-propionyl-4-butyrylphloroglucinol:
[0033]In a 250ml three-neck flask equipped with a stirring device and a reflux condenser, add 5g (0.027mol) 2-propionylphloroglucinol, and under nitrogen protection, slowly add 5.4ml (0.033mol, 5.22g) butyric anhydride and 12.1 ml (13.65g) of boron trifluoride-diethyl ether solution, in which boron trifluoride is 0.096mol, and 10ml of anhydrous diethyl ether is added, and the above reactants are stirred and reacted in an ice-salt water bath for 24h. After the reaction is over, add 100ml of distilled water, stir for 5-10min, separate the reaction solution, extract the water layer with 50ml of ethyl acetate, combine the ester layer with the reaction solution, distill off the solvent under reduced pressure, and recrystallize the obtained solid with 60% ethanol / water The crude product was obtained. Crude yield was 77%. Using a developer o...
Embodiment 3
[0036] As described in Example 1, the difference is that 0.16 mol of phloroglucinol, 0.085 mol of propionic anhydride, and 0.94 mol of boron trifluoride (ether solution) were added. The crude product yield was 67.2%. The crude product was recrystallized to obtain a pure product yield of 59.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com