Phloroglucinol acetyl derivative and its synthesis method and uses
An acylation and reaction technology, applied in the field of phloroglucinol acetyl derivatives and synthesis thereof, can solve problems such as no reports on biological activity testing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
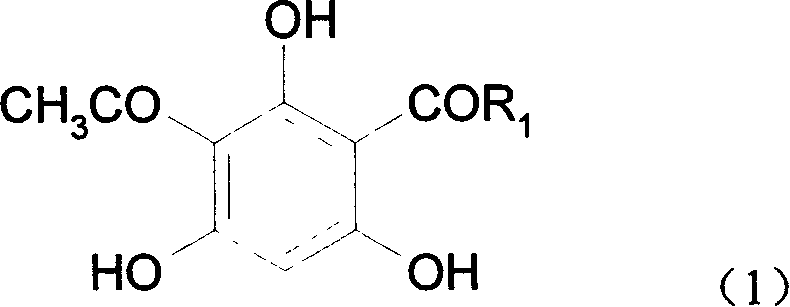
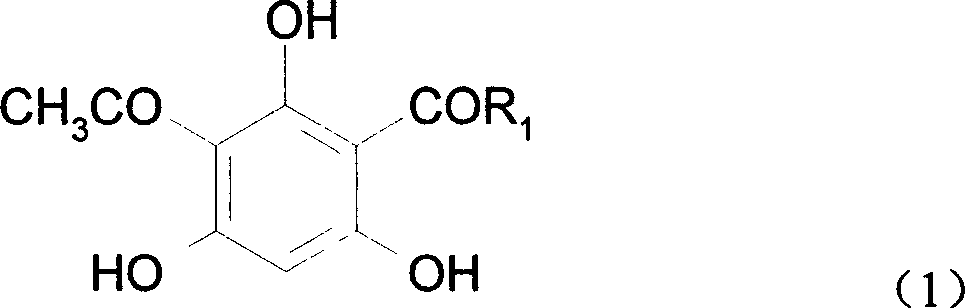
[0017] 2-Acetyl-4-propionylphloroglucinol, the molecular formula is:
[0018]
[0019] Synthesis of 2-acetyl-4-propionylphloroglucinol:
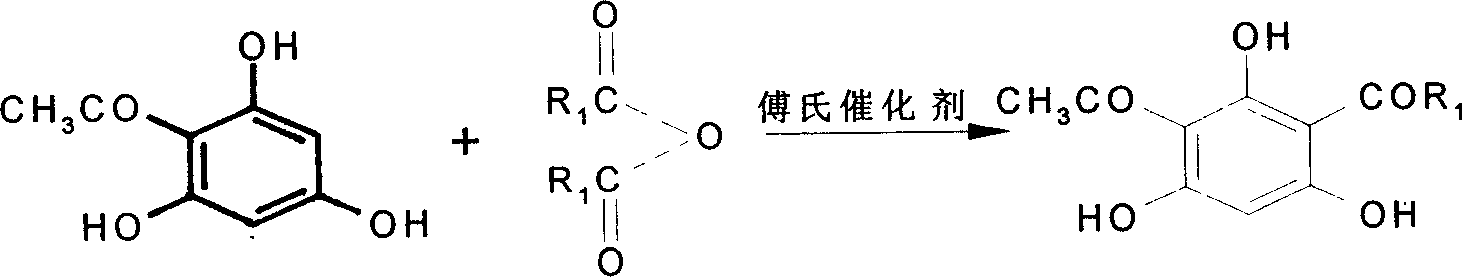
[0020] In a 250ml three-neck flask equipped with a stirring device and a reflux condenser, add 10g (0.060mol) 2-acetylphloroglucinol, and slowly add 9.2ml (0.071mol, 9.30g) propionic anhydride dropwise under nitrogen protection, Add 22.6ml (25.5g) of boron trifluoride-diethyl ether solution dropwise under stirring condition, including 0.18mol of boron trifluoride. . After the reaction is over, add 100ml of distilled water, stir for 5-10min, separate the reaction solution, extract the water layer with 50ml of ethyl acetate, combine the ester layer with the reaction solution, distill off the solvent under reduced pressure, and recrystallize the obtained solid with 60% ethanol / water The crude product was obtained, and the yield of the crude product was 78.9%. Using a developer of 3:1 n-hexane / ethyl acetate solution, the above crude produc...
Embodiment 2
[0023] 2-Acetyl-4-butyrylphloroglucinol, the molecular formula is:
[0024]
[0025] Synthesis of 2-acetyl-4-butyrylphloroglucinol:
[0026] In a 250ml three-necked flask equipped with a stirring device and a reflux condenser, add 5g (0.03mol) of 2-acetylphloroglucinol, and place the three-necked flask in an ice-salt bath so that the reaction temperature is not higher than 5°C, slowly Add 5.8ml (0.036mol, 5.65g) of butyric anhydride, then add 13.1ml (14.79g) of boron trifluoride-ether solution, in which boron trifluoride is 0.104mol, after the dropwise addition, add 10ml of anhydrous ether to make the reaction The substance was completely dissolved, and the reaction temperature was kept at -2-2°C and stirred for 24 hours. After the reaction, add 15ml of distilled water, stir vigorously, then add 30ml of 1:1 methanol / water, carefully add 10gK 2 CO 3 , make the solution pH=7-8, then add 10ml of 1:1 methanol / water, stir at room temperature for 6h, add 10% hydrochloric acid ...
Embodiment 3
[0029] As described in Example 1, the difference is that 0.060 mol of 2-acetylphloroglucinol, 0.085 mol of propionic anhydride, and 0.24 mol of boron trifluoride (diethyl ether solution) were added. The crude product yield was 82.4%, and the crude product was separated to obtain a pure product yield of 68%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com