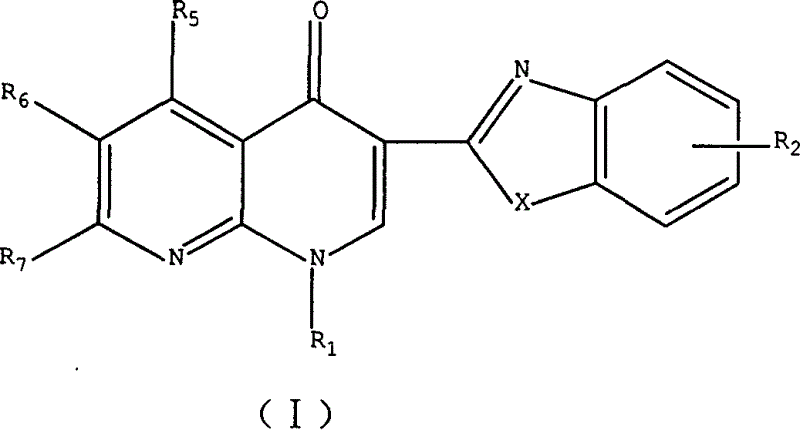
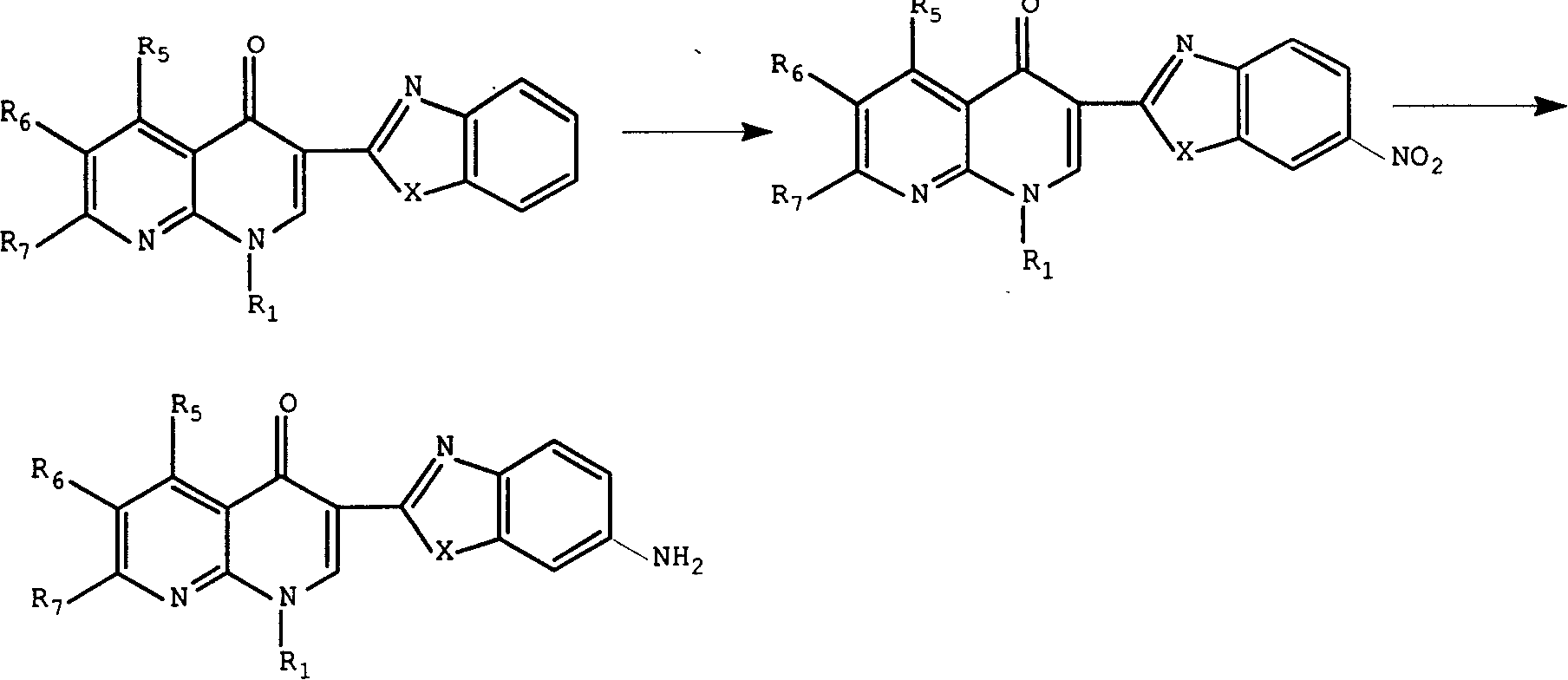
3-substituted nalidixic acid analog compound and its preparation method and uses in pharmacy
The technology of a kind of compound, naphthyridone, is applied in the application field in the preparation antitumor drug
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1: 1-ethyl-3-(2-benzoimidazolyl)-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-1,8-naphthalene Pyridine (1)
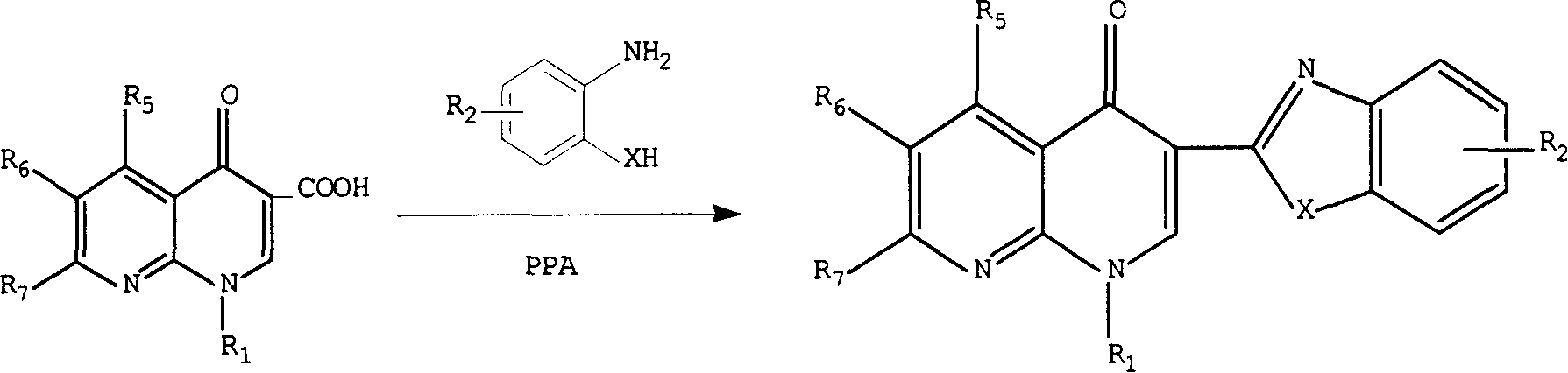
[0064]Enoxacin (3.19 g, 0.01 mol) and o-phenylenediamine (1.08 g, 0.01 mol) were mixed and ground in a mortar, added to the reaction bottle, 40 ml of polyphosphoric acid was added, and the air in the reaction bottle was removed under reduced pressure. When no bubbles are generated, feed nitrogen gas, stir slowly to 140°C and keep warm until the reaction solution is homogeneous, then raise the temperature to 180°C and react for 4 hours, then cool down to about 100°C, pour the reaction solution into 250 grams of crushed ice while stirring , set aside to cool, adjust the pH value to 9, filter with suction, and dry to obtain the crude product, recrystallize with DMF, and then use methanol-triethylamine as the developing solvent for column chromatography to obtain 0.90 g of off-white crystals, which are recrystallized with methanol. Column chromatography gave...
Embodiment 2
[0071] Example 2: 1-ethyl-3-(2-benzoxazolyl)-6-fluoro1,4-dihydro-4-oxo-7-(1-piperazinyl)-1,8-naphthalene Pyridine (2)
[0072] Using enoxacin and o-aminophenol as raw materials, the preparation method is the same as in Example 1, and recrystallized with DMF. Column chromatography gave a light yellow solid, yield 28.9%, mp233-5°C
[0073] IR(cm-1): 3412, 3289, 2981, 1637, 1473, 1445, 1265, 792
[0074] 1HNMR (δ, ppm, DMSO-d6):
[0075] 1.40(t, 3H, -CH3), 2.85(m, 4H, -CH2-*2), 3.67(d, 4H, -CH2-*2), 4.41(q, 2H, -CH2-), 7.37(m , 2H, 5`-H and 6`-H), 7.71(m, 2H, 4`-H and 7`-H), 7.98(d, 1H, 5-H), 8.93(s, 1H, 2- h)
[0076] Formula: C21H20N502F MW: 393.42
[0077] Anal (C%, H%, N%,) Calc: 64.11, 5.12, 17.80 Found: 63.76, 5.34, 17.97
[0078] MS(EI, m / s): 393(M+), 351(base peak)
Embodiment 3
[0080] 1-Ethyl-3-(2-benzothiazolyl)-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-1,8-naphthyridine[1- Ethyl-3-(benzothiazol-2-yl)-6-fluro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-1,8-naphthyridine](IVf)
[0081] Using enoxacin and o-aminothiophenol as raw materials, the preparation method is the same as in Example 1, and recrystallized with methanol. Column chromatography gave a yellow solid, mp 257-9°C, yield 26.6%.
[0082] IR(cm-1): 3419, 1627, 1474, 1446, 1372, 1276, 792
[0083] 1HNMR (δ, ppm, DMSO-d6):
[0084] 1.44(t, 3H, -CH3), 2.87(m, 4H, -CH2-*2), 3.71(m, 4H, -CH2-*2), 4.53(q, 2H, -CH2-), 7.37(t , 1H, 5`-H), 7.50(t, 1H, 6`-H), 7.96(d, 1H, 7`-H), 8.07(m, 2H, 5-H and 4`-H), 9.24 (s, 1H, 2-H)
[0085] Formula: C21H20N50SF.H20 MW: 427.49
[0086] Anal (C%, H%, N%,) Calc: 59.00, 5.15, 16.38 Found: 58.08, 4.77, 16.26
[0087] MS (EI, m / s): 409 (M+), 367 (base peak).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com