Injectable liquid formulation of paracetamol
A technology for paracetamol and injection, which can be used in non-central analgesics, medical preparations containing active ingredients, antipyretics, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1 liquid pharmaceutical preparation of the present invention and the analysis of this preparation with high performance liquid chromatography
[0033] Formulations 001, 002, 003, 004, 005 and 006 were prepared by mixing paracetamol, ultrapure water for injection, optionally (formulation 004) propylene glycol, buffers (phosphate buffer, phosphate-citric acid salt buffer or citrate buffer) and isotonic agent (sodium chloride); the resulting mixture was heated at 70-90°C for about 15 minutes to avoid recrystallization of paracetamol due to the presence of crystallization nuclei; into a glass bottle, and sterilize the glass bottle at 121°C for 15 minutes.
[0034] According to the method recommended by the European Pharmacopoeia (European Pharmacopoeia 4.4, pp.3503-4, 04 / 2003: 0049Paracetamol), the preparation solution before and after sterilization is analyzed by high-performance liquid chromatography. The determination conditions of high-perf...
Embodiment 2 2
[0042] The research of embodiment 2 dimer formation conditions
[0043] 1) The influence of temperature and time
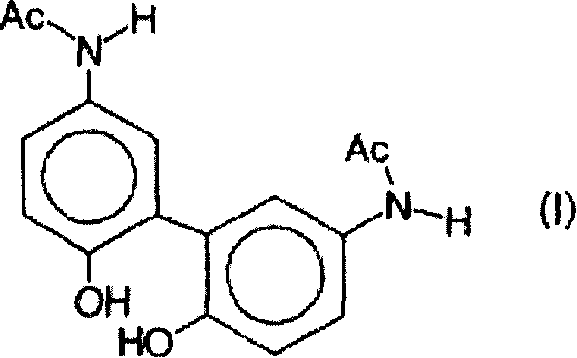
[0044] After preparation 001 in Example 1 was heated at 70-90° C. for 15 minutes and stored in a glass bottle, the influence of temperature on the formation of paracetamol dimer represented by structural formula (I) was investigated. This formulation was in turn stored at different temperatures and / or subjected to different sterilization times and analyzed by HPLC as described in Example 1.
[0045] The main results are shown in Table 2.
[0046] Paracetamol (%)
p-Aminophenol(%)
Dimer (%)
Store at 70°C for 1 hour (non-sterile)
100.8
not detected
not detected
Store at 60°C for 14 days (non-sterile)
100.2
not detected
0.10
Sterilize at 121°C for 10 minutes
99.9
not detected
0.09
Sterilize at 121°C for 20 minutes
99.7
not detected
0.18
After sterilizat...
Embodiment 3
[0051] Embodiment 3 stability study
[0052] After the 001 preparation in Example 1 was heated at 70-90°C for 15 minutes and packed in a glass bottle or a polypropylene infusion container, it was sterilized in a glass bottle at 121°C for 15 minutes or in a polypropylene container at 120°C. The stability was examined after sterilization at ℃ for 20 minutes.
[0053] With the high performance liquid chromatography analysis described in embodiment 1, the results are shown in Table 3.
[0054] 50ml glass bottle
100ml polypropylene container
start
state
10 months later
start
state
10 months later
25℃
40℃
25℃
40℃
Osmolality
(mOsm / kg)
283
288
285
281
289
283
Paracetamol content (%)
100.3
100.7
99.1
100.3
100.4
99.4
Dimer content (%)
0.11
0.23
0.73
0.09
0.14
0.47 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com