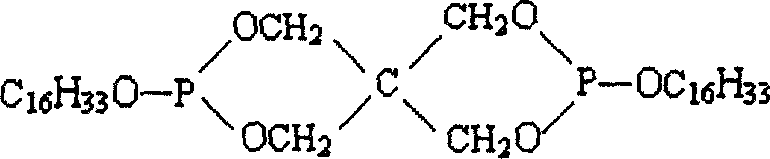
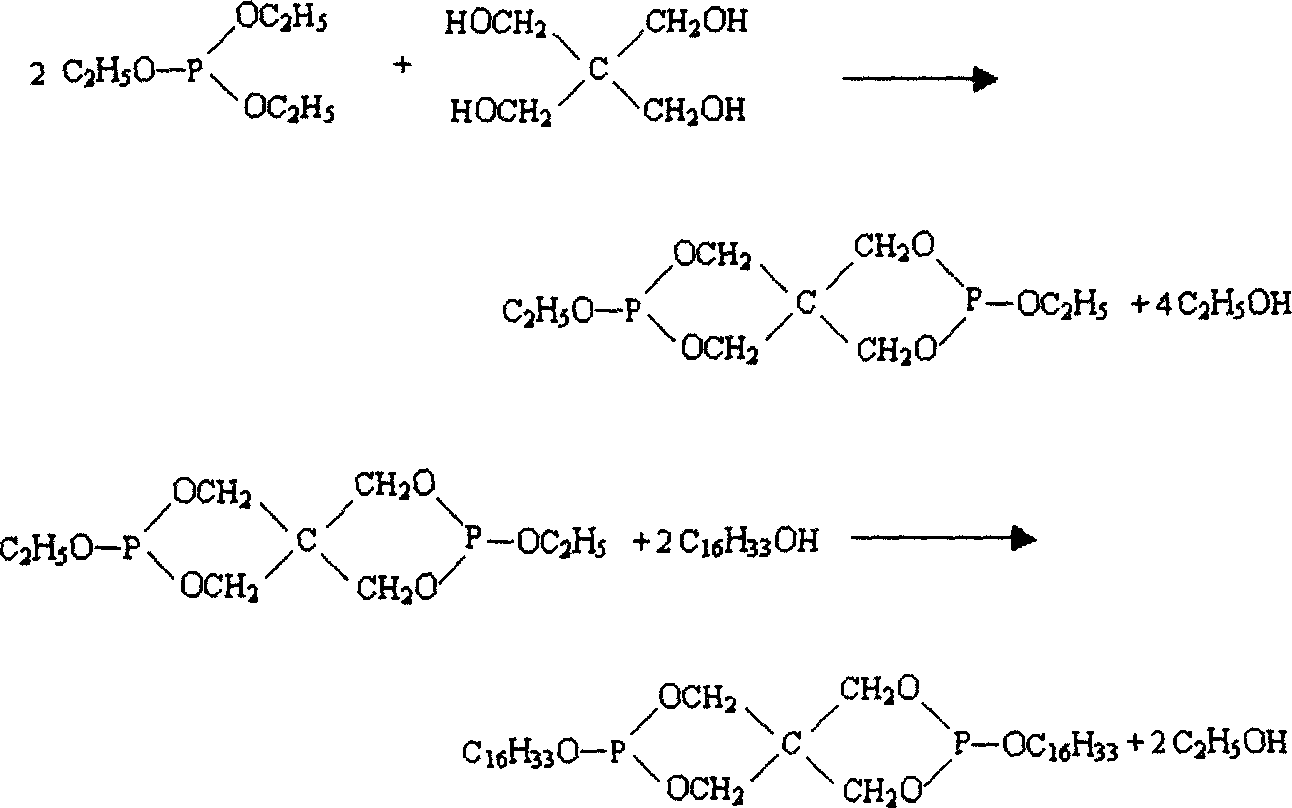
Dicetylpentaerythritoldiphosphite ester synthesis method
A technology of alkyl pentaerythritol diphosphite and pentaerythritol, which is applied in the field of synthesis of dihexadecyl pentaerythritol diphosphite, and can solve the problems of low yield, cumbersome post-treatment process, complicated solvent recovery and purification, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The synthetic method of dihexadecyl pentaerythritol diphosphite comprises the following steps:
[0025] 1. Add pentaerythritol, triethyl phosphite and catalyst into a three-necked flask equipped with a stirrer, condenser, thermometer and receiver, and stir at a stirring speed of 300 rpm; heat and react for a period of time under specified temperature conditions , at a temperature of 130°C and a pressure of -0.02Mpa, the ethanol generated by the reaction and unreacted triethyl phosphite are distilled off under reduced pressure to prepare pure pentaerythritol diphosphite;
[0026] Wherein, the mol ratio of triethyl phosphite and pentaerythritol is 2.0: 1;
[0027] By weight, the consumption of the catalyst is 1% of the amount of pentaerythritol; the catalyst can be sodium methylate;
[0028] The reaction temperature is 100°C; the reaction time is 0.5h;
[0029] 2, in the reaction product that (1) step obtains, need not separate and purify, directly add cetyl alcohol whe...
Embodiment 2
[0033] The synthetic method of dihexadecyl pentaerythritol diphosphite comprises the following steps:
[0034] 1. Add pentaerythritol, triethyl phosphite and catalyst into a three-necked flask equipped with a stirrer, condenser, thermometer and receiver, and stir at a stirring speed of 500 rpm; heat and react for a period of time under specified temperature conditions , at a temperature of 130°C and a pressure of 0.02Mpa, the ethanol generated by the reaction and unreacted triethyl phosphite are distilled off under reduced pressure to prepare pure pentaerythritol diphosphite;
[0035] Wherein, the mol ratio of triethyl phosphite and pentaerythritol is 2.20: 1;
[0036] By weight, the consumption of the catalyst is 3% of the amount of pentaerythritol; the catalyst can be sodium hydroxide;
[0037] The reaction temperature is 130°C; the reaction time is 2.5h;
[0038] 2, in the reaction product that (1) step obtains, need not separate and purify, directly add cetyl alcohol whe...
Embodiment 3
[0042] The synthetic method of dihexadecyl pentaerythritol diphosphite comprises the following steps:
[0043] 1. Add pentaerythritol, triethyl phosphite, and catalyst to a three-necked flask equipped with a stirrer, condenser, thermometer, and receiver, and stir at a stirring speed of 700 rpm; heat and react for a period of time under specified temperature conditions , at a temperature of 130°C and a pressure of 0.06Mpa, the ethanol and unreacted triethyl phosphite generated by the reaction were distilled off under reduced pressure to prepare pure pentaerythritol diphosphite;
[0044] Wherein, the mol ratio of triethyl phosphite and pentaerythritol is 2.40: 1;
[0045] By weight, the consumption of catalyst is 4% of pentaerythritol amount;
[0046] The catalyst can be organotin; the reaction temperature is 150°C; the reaction time is 3h;
[0047] 2, in the reaction product that (1) step obtains, need not separate and purify, directly add cetyl alcohol wherein, stir, stirrin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com