Group-B type-I Coxsackie virus gene vaccine
A coxsackie virus and gene vaccine technology, applied in the field of vaccines to prevent coxsackie virus infection, can solve the problems of poor safety and poor immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
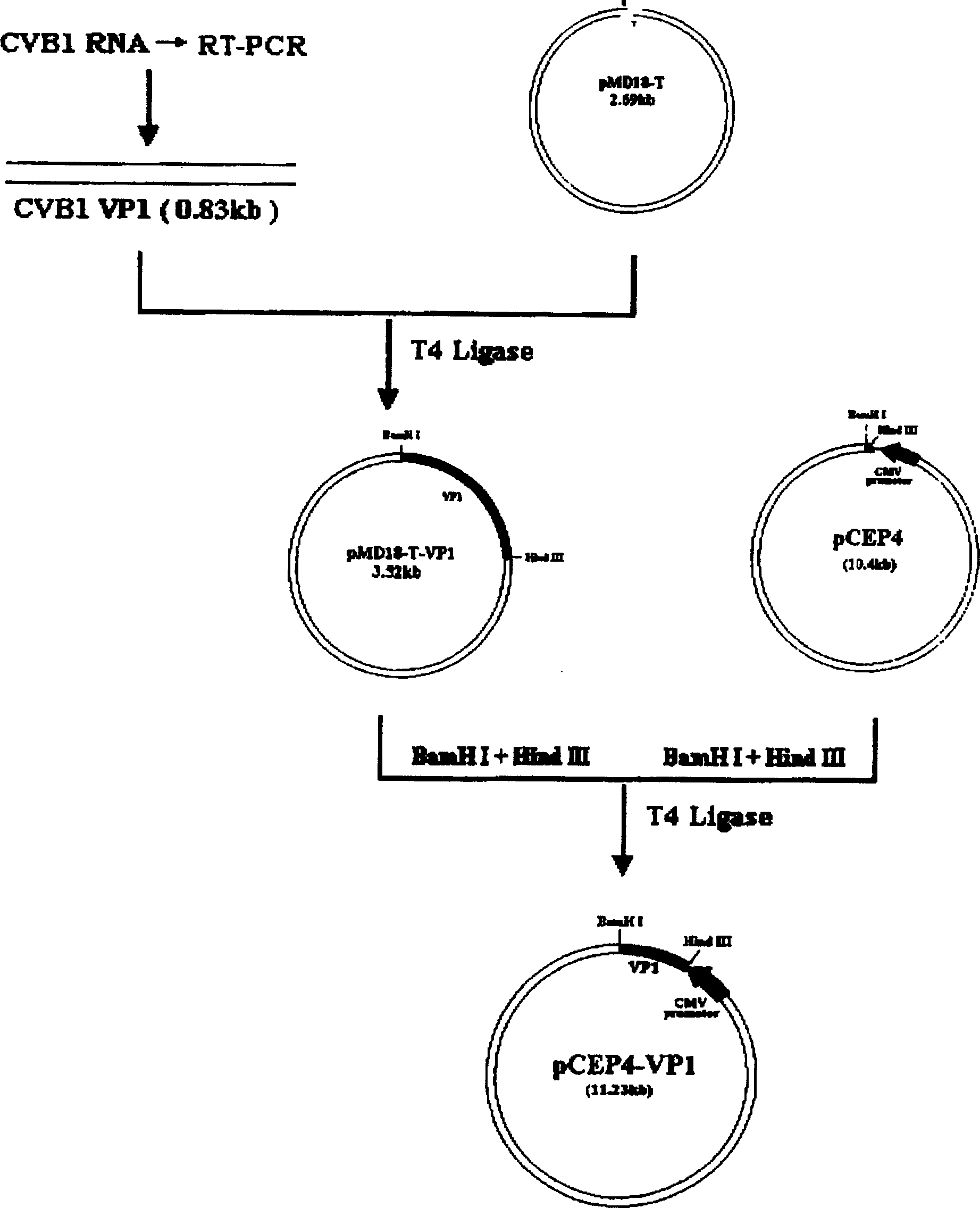
[0006] Embodiment 1: The CVB1 gene vaccine of this embodiment is a pCEP4-CVB 1VP1 plasmid consisting of the VP1 gene encoding the main neutralizing antigen of CVB1 and the plasmid pCEP4 as a eukaryotic expression vector. For the gene sequence of CVB1VP1, see the gene sequence table.
specific Embodiment approach 2
[0007] Specific implementation mode 2: This implementation mode introduces the CVB1 gene vaccine in detail.
[0008] 1. Materials
[0009] 1.1 Viruses and cells: CVB1 virus strain, Vero cells, HeLa cells and P815 cells were provided by the second diagnostic department of the Institute of Virology, Chinese Academy of Preventive Medicine. s MEM was subcultured, and P815 cells were cultured in RPMI1640 medium.
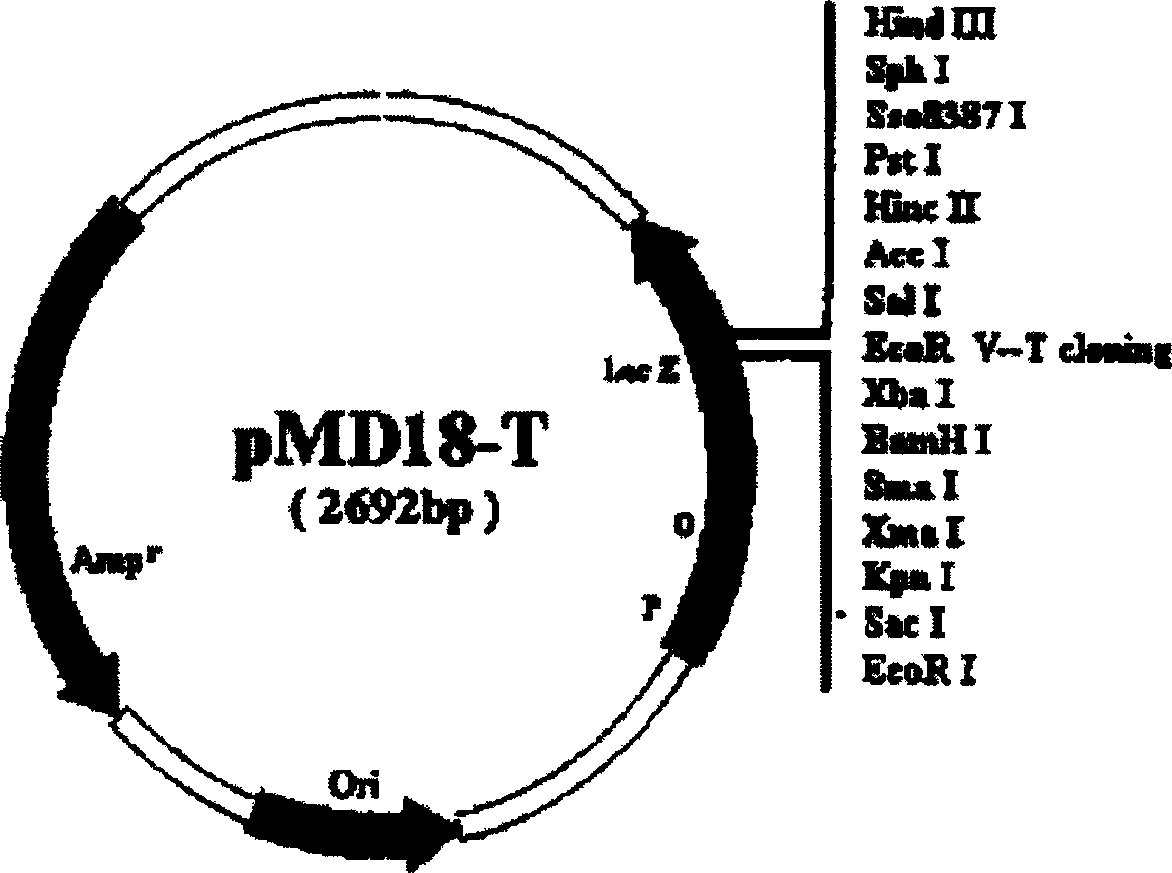
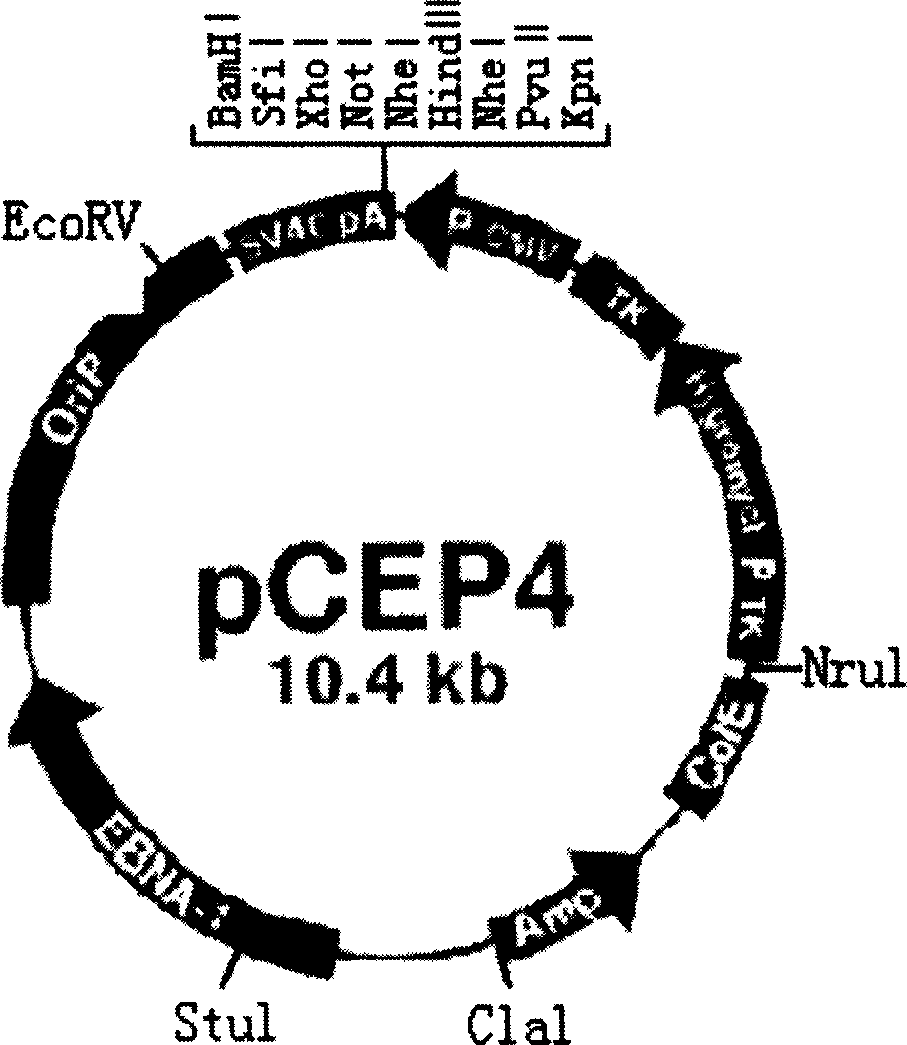
[0010] 1.2 Vectors and strains: The vectors used in the experiment included pMD18-T, pCEP4, and the host strain of pMD18-T and pCEP4 was JM109. pMD18-T is a product of TaKaRa Biotechnology Company, with a size of 2.7kb. It is a high-efficiency TA cloning vector and is suitable for PCR product cloning. Screen for recombinants. In addition, pMD18-T has M13forward Primer and M13reverse primer at both ends of the multiple cloning site, which is convenient for sequencing the cloned fragments (see figure 1 ). pCEP4 is a product of Invitrogen Company, with a size of 10.4kb....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com