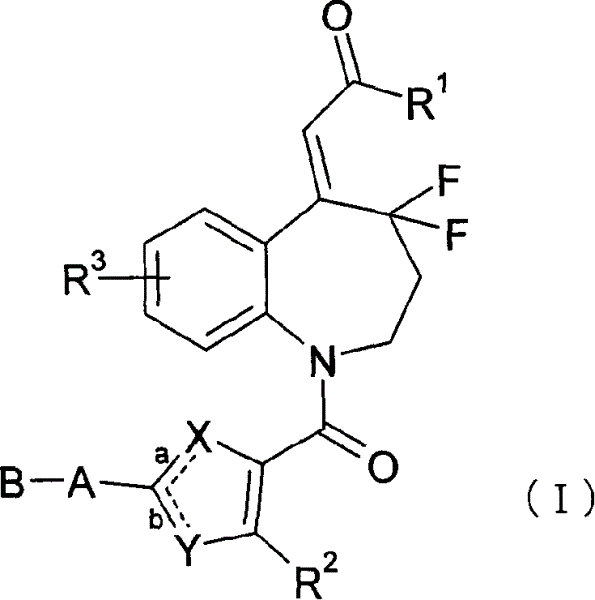
4,4-difluoro-1,2,3,4-tetrahydro-5h-1-benzoazepine derivative or salt of the same
A technology of benzazepines, compounds, applied in the field of medicine using said compounds as active ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0331] 150 mg of the compound of Reference Example 20 was dissolved in 5 ml of DMF, mixed with 43 mg of HOBt, 61 mg of WSCD, 35 mg of glycinamide hydrochloride and 0.045 ml of triethylamine, followed by stirring at room temperature for 4 hours. To the reaction mixture were added saturated aqueous sodium bicarbonate solution and EtOAc, and the layers were separated. The organic layer was washed with water and saturated brine, and dried over anhydrous magnesium sulfate. The solvent was evaporated and the obtained residue was recrystallized from EtOH to give 139 mg of (2Z)-N-(2-amino-2-oxoethyl)-2-{1-[4-(benzyloxy)-2- (Trifluoromethyl)benzoyl]-4,4-difluoro-1,2,3,4-tetrahydro-5H-1-benzazepine-5-ylidene}acetamide.
[0332] In the same manner as in Example 1, using the corresponding raw materials, respectively, Examples 2 to 16 shown in Table 10 were prepared.
Embodiment 17
[0334] 150 mg of the compound of Example 20 was dissolved in 3.5 ml of THF, mixed with 0.3 ml of thionyl chloride and 2-3 drops of DMF, and stirred at room temperature for 1 hour. The solvent was evaporated under reduced pressure, and thionyl chloride was further removed by azeotropic distillation using toluene. The obtained residue was dissolved in THF, and the solution was added dropwise to ammonia water. EtOAc was added to the reaction mixture, and the layers were separated. The organic layer was washed with saturated brine, and dried over anhydrous magnesium sulfate. The obtained crude product was recrystallized in iPrOH-diisopropyl ether mixed solvent to obtain 126 mg of (2Z)-2-{1-[4-(benzyloxy)-2-(trifluoromethyl)benzoyl]- 4,4-Difluoro-1,2,3,4-tetrahydro-5H-1-benzazepine-5-ylidene}acetamide.
[0335] In the same manner as in Example 17, using the corresponding starting materials, Example 18 shown in Table 11 was prepared. In addition, in the same manner as in Referen...
Embodiment 21
[0337] 325 mg of the compound of Example 6 was dissolved in 5 ml of 1,2-dichloroethane, mixed with 148 mg of m-chlorobenzoic acid under ice-cooling, and stirred at room temperature for 4 hours. The reaction mixture was mixed with 10% (w / v) Na 2 S 2 O 3 ·5H 2O aqueous solution, water and chloroform were mixed, and the layers were separated. The organic layer was washed with saturated aqueous sodium bicarbonate solution, dried over anhydrous sodium sulfate, and the solvent was evaporated. The obtained crude product was subjected to silica gel column chromatography, eluted with chloroform-MeOH (23:2), and concentrated under reduced pressure to obtain 121 mg ( 2Z)-N-(2-amino-2-oxoethyl)-2-{4,4-difluoro-1-[4-(propylsulfinyl)benzoyl]-1,2,3 , 4-tetrahydro-5H-1-benzazepine-5-ylidene}acetamide.
[0338] Example 22 shown in Table 10 was prepared in the same manner as Example 21 using the corresponding starting materials. In addition, using the methods described above or the method...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com