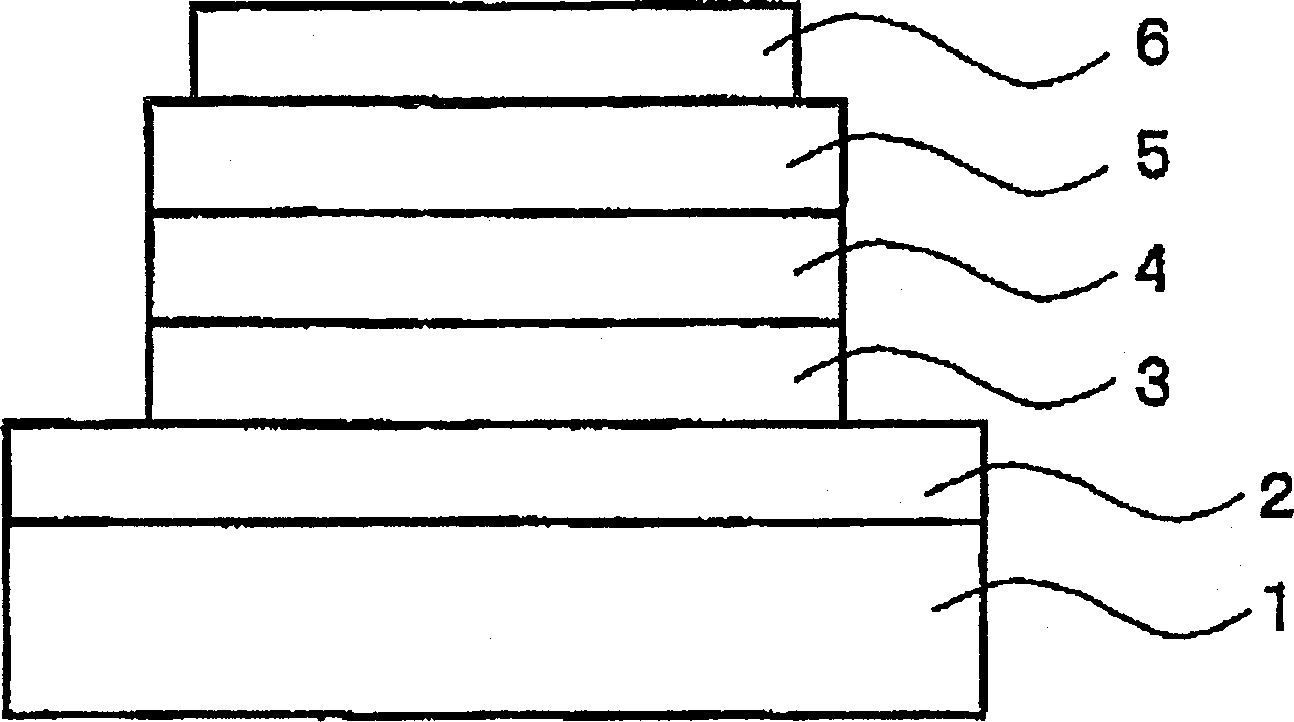
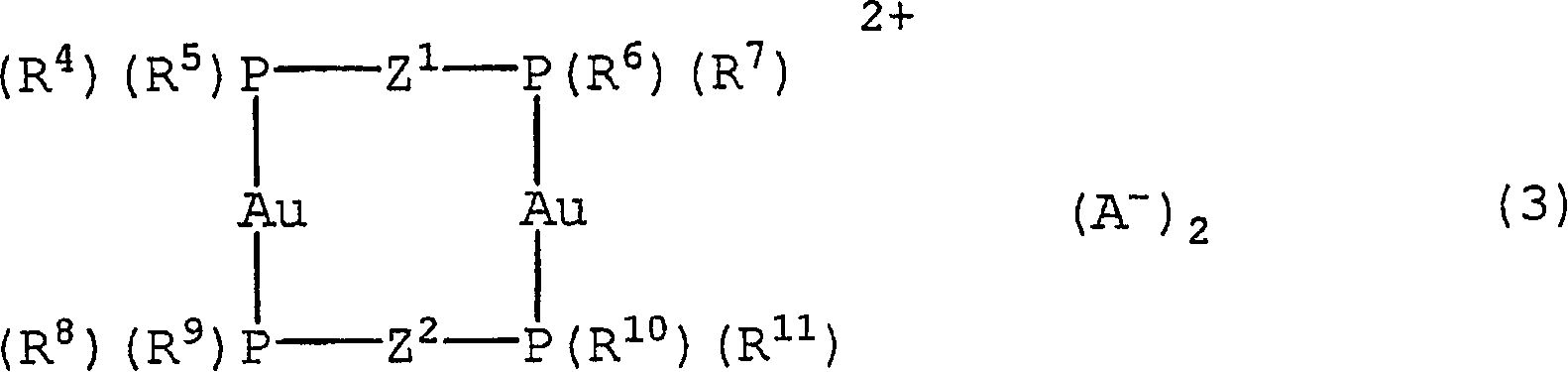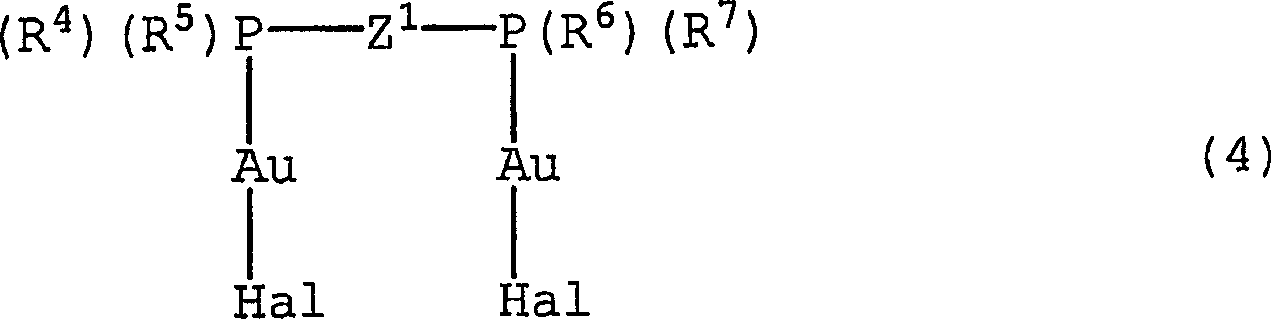Organic polymer light-emitting element material having gold complex structure and organic polymer light-emitting element
By using phosphorescent coordination compounds with a gold complex structure and polymers with polymerizable functional groups in organic EL devices, the development problems of multi-color or white light emitting materials and polymer stability problems in the existing technology are solved, and the problem of polymer stability is achieved. Efficient multicolor or white light emission and large-area fabrication.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0149] Embodiment 1: the synthesis of polymerizable gold complex (1)
[0150]
[0151]
[0152] The polymerizable gold complex (1) was synthesized by reacting sodium tetrachloroaurate (III) with thiodiglycol and then with bis(diphenylphosphino)methane containing a polymerizable functional group. More specifically, 20 ml of tetrahydrofuran (hereinafter abbreviated as "THF") was added to 20 g (5.2 mmol) of bis(diphenylphosphino)methane, and while cooling on ice, a solution of n-butyllithium was added dropwise. 3.5 mL (5.6 mmol) of 1.6M hexane solution. After adding a THF solution containing 10 mg (0.05 mmol) of lithium tetrachlorocuprate to the prepared slurry, 10 mL of a THF solution containing 0.85 g (5.6 mmol) of 4-vinylbenzyl chloride was added dropwise , and stirred at room temperature for 3 hours. Thereafter, the solvent was distilled off under reduced pressure, and the organic material was extracted with dichloromethane, and then dried under reduced pressure. The...
Embodiment 2
[0158] Embodiment 2: Synthesis of polymerizable gold complex (2)
[0159]
[0160] The polymerizable gold complex (2) was synthesized by reacting the polymerizable gold complex (1) with bis(diphenylphosphino)methane. More specifically, 150 mg (0.20 mmol) of gold complex (1) was dissolved in 10 ml of dichloromethane, and 77 mg (0.20 mmol) of bis(diphenylphosphino)methane and 100 mg ( 0.39 mmol) silver trifluoromethanesulfonate, and stirred at room temperature for 2 hours. After distilling off the solvent under reduced pressure, the resulting reaction mixture was extracted with dichloromethane, and then dried under reduced pressure. The residue was dissolved in a small amount of dichloromethane, concentrated after further adding methanol, and the resulting crystals were collected by filtration and dried to obtain 118 mg (0.096 mmol) of the target polymerizable gold complex (2). It was identified by CHN elemental analysis and mass spectrometry.
[0161] Elemental analysis: ...
Embodiment 3
[0165] Embodiment 3: Synthesis of polymerizable gold complex (3-1)
[0166]
[0167]
[0168] A gold complex obtained by reacting sodium tetrachloroaurate (III) with triphenylphosphine containing a polymerizable functional group was reacted with a known method (see, P. Cadiot and W. Chodkiewicz, Chemistry of Acetylenes, H.G. Viehe, ed., Marcel Dekker, New York (1969)) reacted with phenylbutadiyne synthesized, thereby synthesizing the polymerizable gold complex (3-1). More specifically, 20 ml of anhydrous THF was added to 341 mg (14 mmol) of magnesium, and 10 ml of a THF solution containing 2.70 g (15 mmol) of 4-bromostyrene was added dropwise thereto to prepare the Grignard reagent . To the resulting solution was added dropwise 10 ml of a THF solution containing 2.50 g (11 mmol) of chlorodiphenylphosphine, and stirred at room temperature for 1.5 hours. After the solvent was distilled off under reduced pressure, the residue was purified by silica gel column chromatograp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com