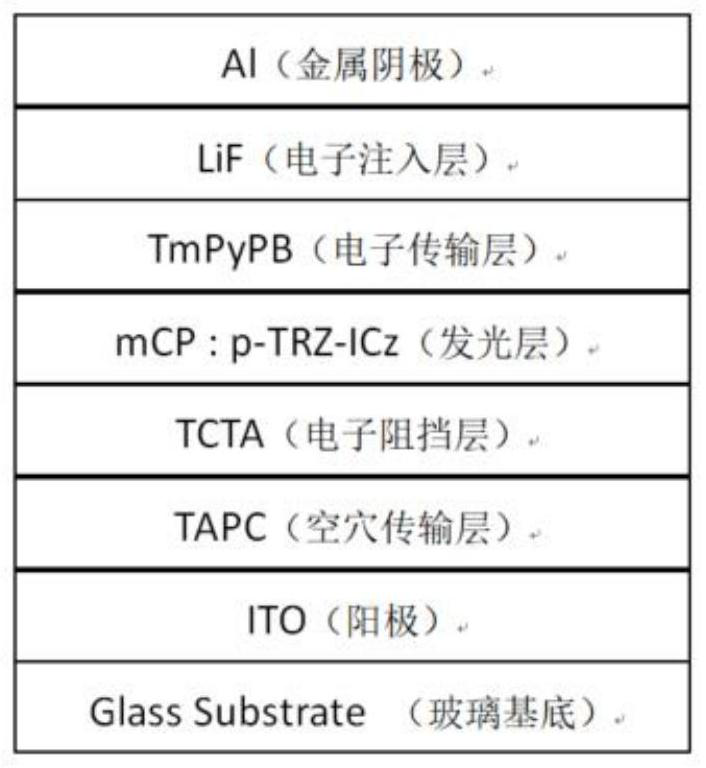
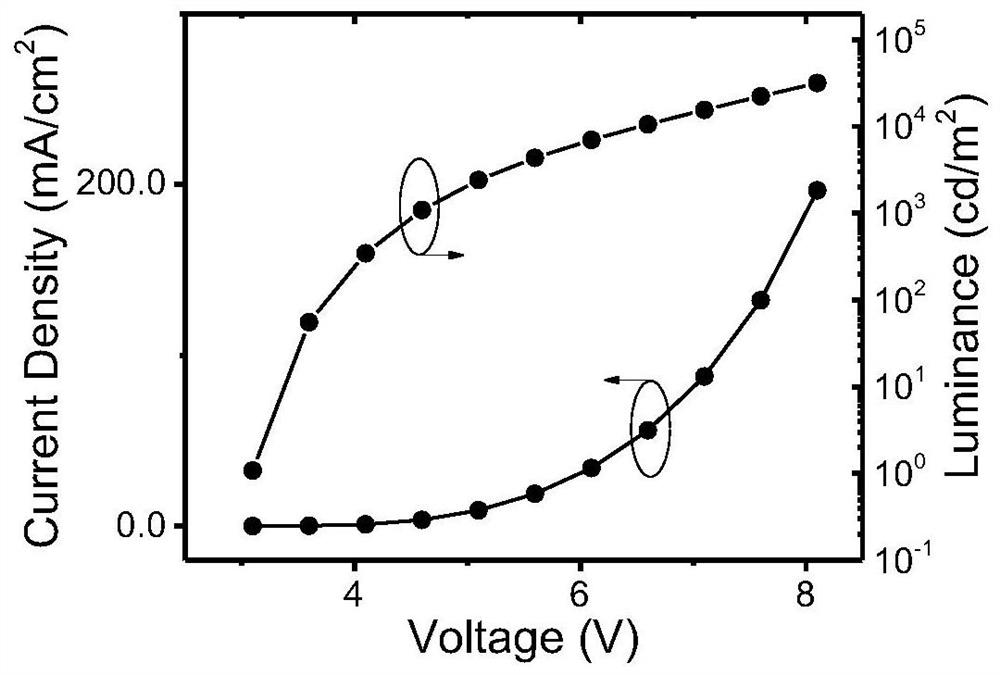
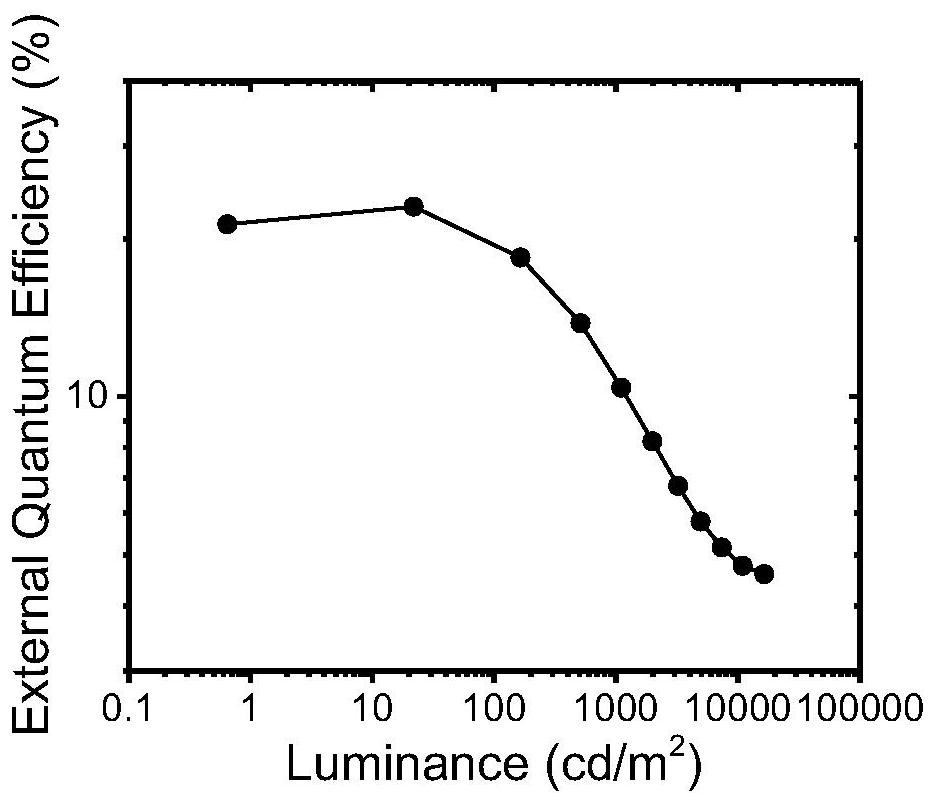
Indolocarbazole-based organic light-emitting material and preparation method and application thereof
A technology of indolocarbazole and luminescent materials, applied in luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of low quantum efficiency, and achieve the effect of simple synthesis method, stable material structure and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] This embodiment proposes an organic light-emitting material (p-TRZ-ICz) based on indolocarbazole, which has the structure shown below:
[0041]
[0042] The synthetic route of the organic luminescent material (p-TRZ-ICz) based on indolocarbazole is as follows:
[0043]
[0044] Specifically include the following steps:
[0045] Indolo[3,2-B]carbazole (0.256g, 1mmol), 2-(4-bromophenyl)-4,6-diphenyl-1,3,5-triazine (0.814g, 2.1mmol), sodium tert-butoxide (0.288g, 3mmol) and palladium acetate (0.022g, 0.1mmol) were added to the reaction flask, pumped and ventilated three times, and tri-tert-butylphosphine (0.061g, 0.3 mmol), finally add 20mL toluene, heat up to 110°C, and react at this temperature for 12 hours. After the reaction was completed, the reaction solid was obtained by suction filtration, recrystallized using dichloromethane and methanol, and the final product p-TRZ-ICz was obtained by suction filtration, with a final yield of 93%.
[0046] This embodimen...
Embodiment 2
[0049] This embodiment proposes an organic light-emitting material (m-TRZ-ICz) based on indolocarbazole, which has the structure shown below:
[0050]
[0051] The synthetic route of the organic luminescent material (p-TRZ-ICz) based on indolocarbazole is as follows:
[0052]
[0053] Specifically include the following steps:
[0054] Indolo[3,2-B]carbazole (0.256g, 1mmol), 2-(3-bromophenyl)-4,6-diphenyl-1,3,5-triazine (0.814g, 2.1mmol), sodium tert-butoxide (0.288g, 3mmol) and palladium acetate (0.022g, 0.1mmol) were added to the reaction flask, pumped and ventilated three times, and tri-tert-butylphosphine (0.061g, 0.3 mmol), finally add 20mL toluene, heat up to 110°C, and react at this temperature for 12 hours. After the reaction was completed, the reaction solid was obtained by suction filtration, recrystallized using dichloromethane and methanol, and the final product m-TRZ-ICz was obtained by suction filtration, with a final yield of 95%.
[0055] This embodimen...
Embodiment 3
[0058] This example proposes an organic light-emitting material (p-TXO-ICz) based on indolocarbazole, which has the structure shown below:
[0059]
[0060] The synthetic route of the organic luminescent material (p-TXO-ICz) based on indolocarbazole is as follows:
[0061]
[0062] Specifically include the following steps:
[0063] Indolo[3,2-B]carbazole (0.256g, 1mmol), 2-bromo-10-thiaxanthone (0.609g, 2.1mmol), sodium tert-butoxide (0.288g, 3mmol) and palladium acetate (0.022g, 0.1mmol) were added to the reaction flask, pumped and ventilated three times, tri-tert-butylphosphine (0.061g, 0.3mmol) was added under the protection of nitrogen, and finally 20mL of toluene was added, and the temperature was raised to 110°C. The reaction was carried out at this temperature for 12 hours. After the reaction was completed, the reaction solid was obtained by suction filtration, recrystallized using dichloromethane and methanol, and the final product p-TXO-ICz was obtained by suc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com