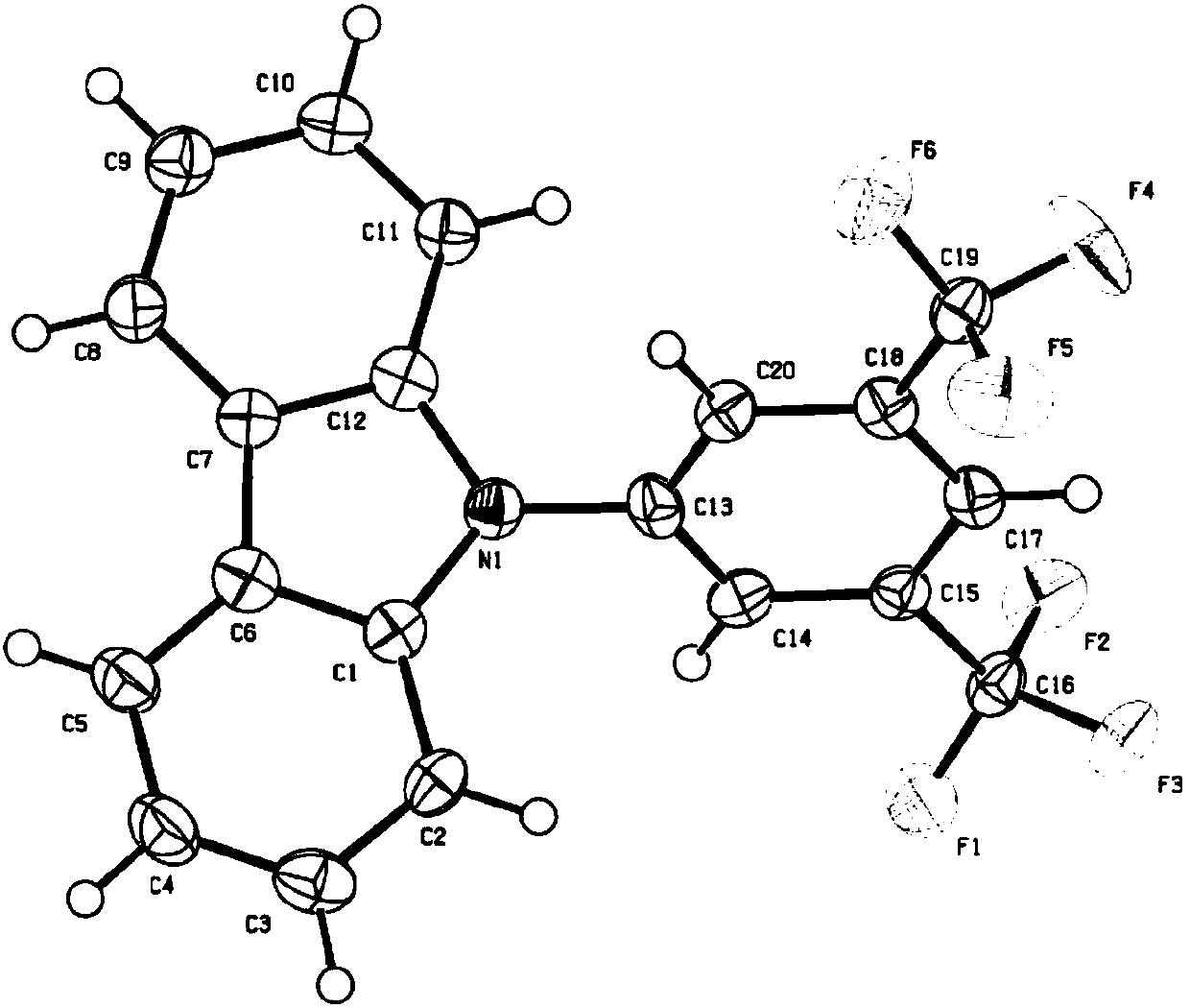
Purple fluorescent material crystal based on trifluoromethyl and preparation method
A trifluoromethyl, violet fluorescence technology, applied in the direction of luminescent materials, organic chemistry methods, chemical instruments and methods, etc., can solve the problem of not many, violet fluorescent material crystals are rare, etc., to achieve small energy level difference, easy to large The effect of large-scale preparation and short material synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: Preparation of 9-[3,5 bis(trifluoromethyl)phenyl]-9H-carbazole
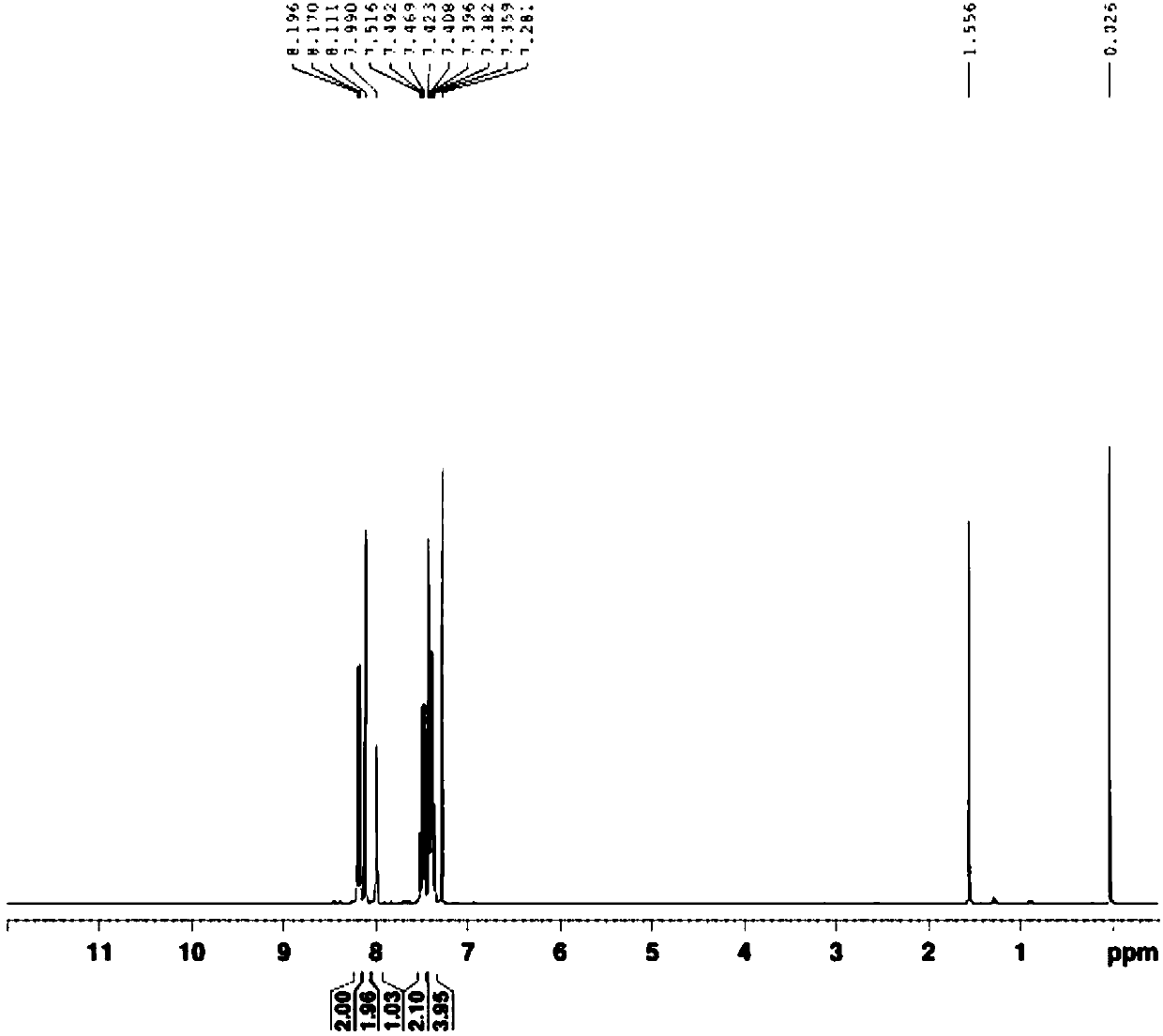
[0025] Add 1-bromo-3,5-bis(trifluoromethyl)benzene 2.91g (10mmol), carbazole 1.80g (10.8mmol), cuprous iodide 0.19g (1mmol), anhydrous potassium carbonate 2.72g (20mmol ), 0.2 g (1 mmol) of 1,10-phenanthroline monohydrate and 73 g (1 mol) of N, N-dimethylformamide were mixed uniformly, and reflux reaction was carried out under nitrogen protection for 40 h. The reaction solution was cooled to room temperature, poured into 100 g of water to precipitate a solid, and after suction filtration, the filter cake was made into sand and subjected to column chromatography, and eluted with petroleum ether with a boiling range of 60-90°C to obtain a white solid with a yield of 63.1%. 1 H NMR (300MHz, CDCl 3 )δ (ppm): 8.18 (d, J = 7.8Hz, 2H), 8.11 (s, 2H), 7.99 (s, 1H), 7.50 (t, J = 7.2Hz, 6.9Hz, 2H), 7.42-7.35 (m, 4H). 1 H NMR spectrum as image 3 shown.
Embodiment 2
[0026] Example 2: Preparation of 9-[3,5-bis(trifluoromethyl)phenyl]-9H-carbazole
[0027] Add 2.91g (10mmol) of 1-bromo-3,5-bis(trifluoromethyl)benzene, 1.67g (10mmol) of carbazole, 0.39g (2mmol) of cuprous iodide, and 2.04g (15mmol) of anhydrous potassium carbonate , 0.40 g (2 mmol) of 1,10-phenanthroline monohydrate and 36.5 g (0.5 mol) of N, N-dimethylformamide were mixed uniformly, and reflux reaction was carried out under nitrogen protection for 20 h. The reaction solution was cooled to room temperature, poured into 50 g of water to precipitate a solid, and after suction filtration, the filter cake was made into sand and subjected to column chromatography, and eluted with petroleum ether with a boiling range of 60-90°C to obtain a white solid with a yield of 53.9%. 1 H NMR (300MHz, CDCl 3 )δ (ppm): 8.18 (d, J = 7.8Hz, 2H), 8.11 (s, 2H), 7.99 (s, 1H), 7.50 (t, J = 7.2Hz, 6.9Hz, 2H), 7.42-7.35 (m, 4H). 1 H NMR spectrum as image 3 shown.
Embodiment 3
[0028] Example 3: Preparation of 9-[3,5-bis(trifluoromethyl)phenyl]-9H-carbazole
[0029] Add 2.91g (10mmol) of 1-bromo-3,5-bis(trifluoromethyl)benzene, 2.0g (12mmol) of carbazole, 0.19g (1mmol) of cuprous iodide, and 2.72g (20mmol) of anhydrous potassium carbonate , 0.20 g (1 mmol) of 1,10-phenanthroline monohydrate and 73 g (1 mol) of N, N-dimethylformamide were mixed uniformly, and refluxed for 20 h under the protection of nitrogen. The reaction solution was cooled to room temperature, poured into 100 g of water to precipitate a solid, and after suction filtration, the filter cake was made into sand and subjected to column chromatography, and eluted with petroleum ether with a boiling range of 60-90°C to obtain a white solid with a yield of 64.2%. 1 H NMR (300MHz, CDCl 3 )δ (ppm): 8.18 (d, J = 7.8Hz, 2H), 8.11 (s, 2H), 7.99 (s, 1H), 7.50 (t, J = 7.2Hz, 6.9Hz, 2H), 7.42-7.35 (m, 4H). 1 H NMR spectrum as image 3 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com