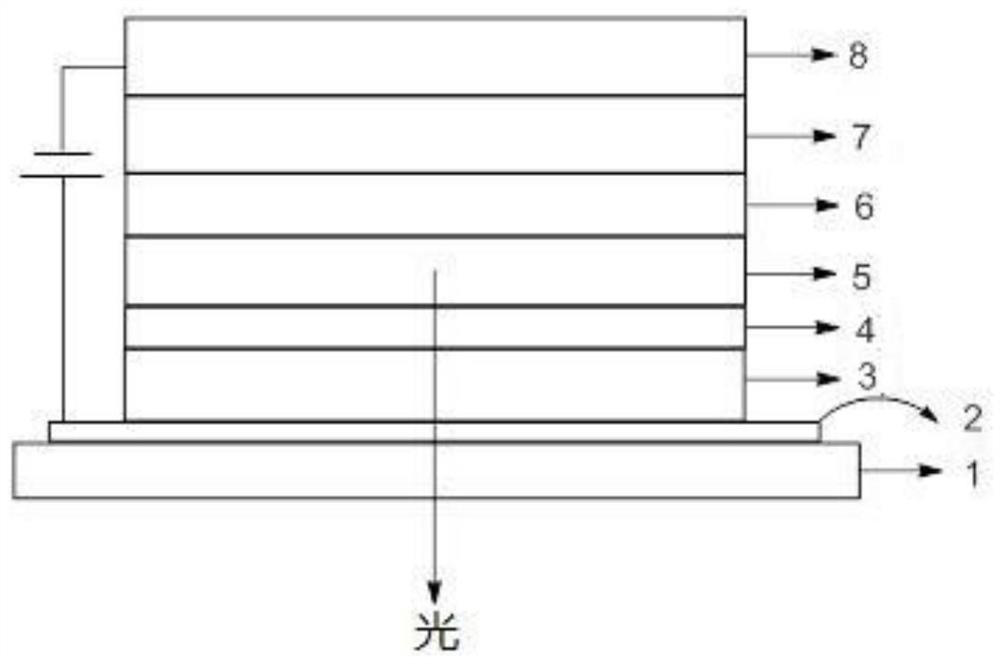
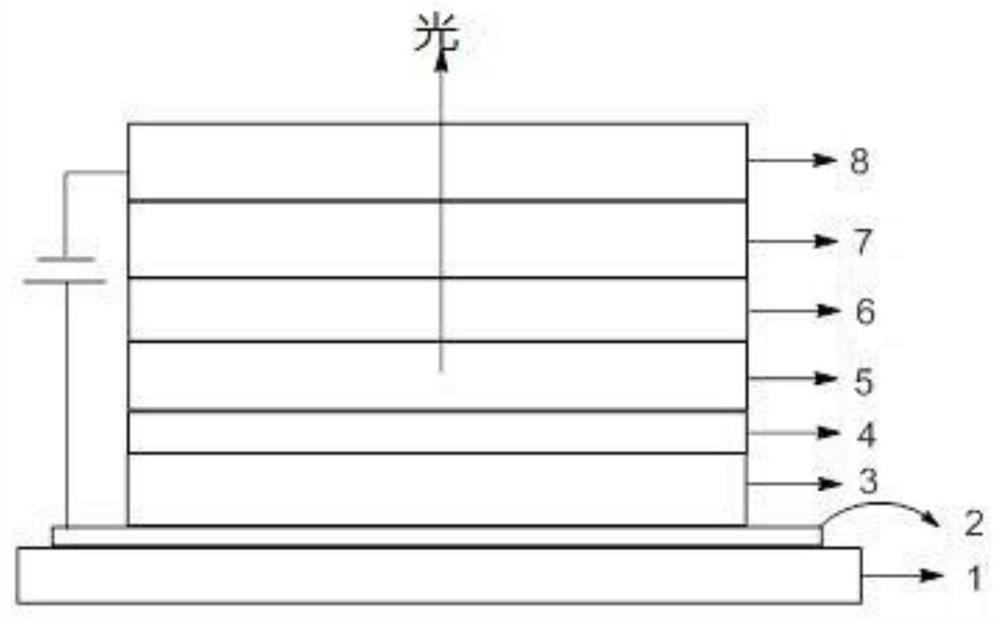
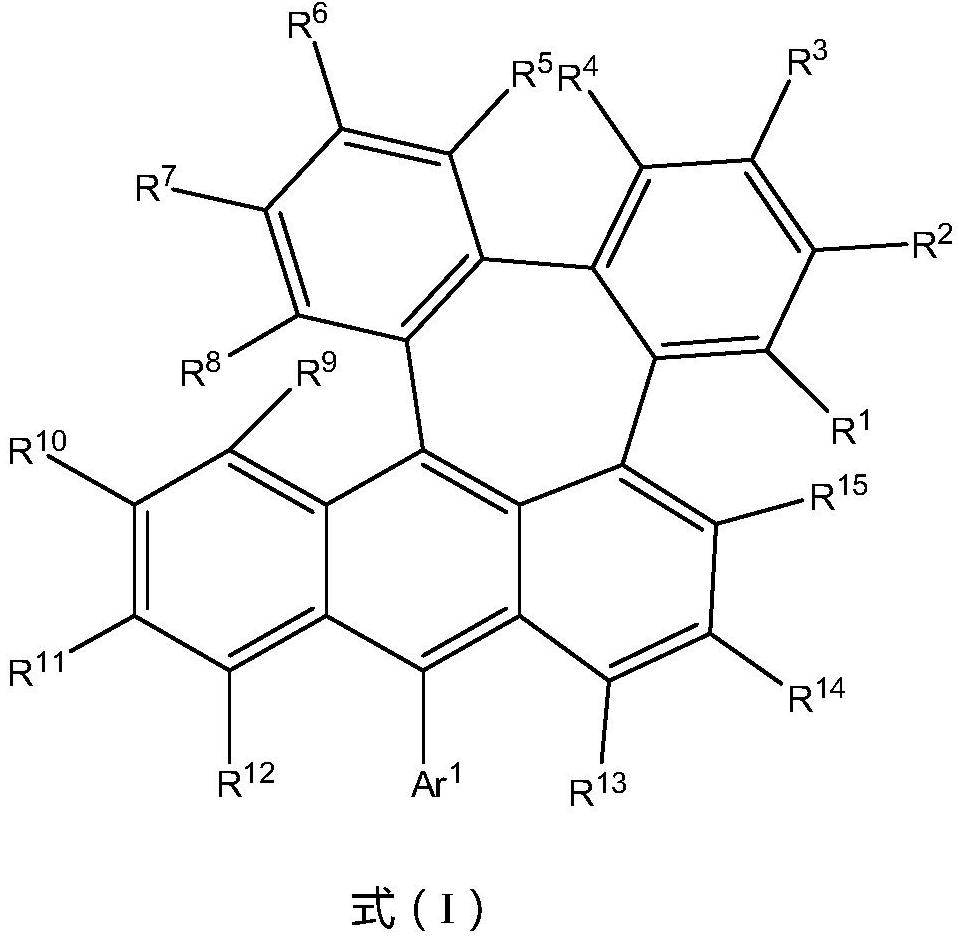
Anthracene derivatives, organic electroluminescent materials, light emitting devices and consumer products
An anthracene derivative and luminescent technology, which is applied in luminescent materials, organic chemistry, circuits, etc., can solve the problems of reducing the quantum yield of the blue light system, aggravating molecular fluorescence quenching, and low luminescent quantum efficiency, so as to improve the internal quantum efficiency , Improve solubility, increase the effect of molecular hindrance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Preparation of intermediate A1:
[0084] The preparation method of intermediate A1, comprises the steps:
[0085] Step 1: Preparation of Compound Int.-1
[0086]
[0087] Under the protection of nitrogen, 50.0mmol of o-iodobromobenzene was dissolved in 100mL of dry tetrahydrofuran, cooled to 0°C, 52.0mL of 1M isopropylmagnesium bromide THF solution was added dropwise, stirred for 1 hour, and 48.0mmol of 9H-Tribenzo[a,c,e][7]cycloann-9-one (CAS:68089-73-6) dissolved in THF solution, raised to room temperature and stirred for 2 hours, added 50mL of 2N dilute Hydrochloric acid aqueous solution was extracted with ethyl acetate, the organic phase was collected, dried, filtered, the filtrate was concentrated and dried under reduced pressure, separated and purified by silica gel column to obtain the intermediate Int.-1 with a yield of 94%.
[0088] The second step: the preparation of compound Int.-2
[0089]
[0090] Under the protection of nitrogen, dissolve 40.0mmol...
Embodiment 2
[0104] Preparation of compound CJHB761:
[0105]
[0106] Mix 15.0mmol of intermediate A1 with 60mL of toluene, and add 18.0mmol of (4-(2-naphthyl)phenyl)boronic acid, 54.0mmol of anhydrous sodium carbonate and 173.0mg of Pd(PPh 3 ) 4 Catalyst, then add 30mL of ethanol and 30mL of water, heat up to reflux and stir for 12 hours, cool to room temperature, filter, wash the filter cake with water and ethanol, separate and purify with silica gel column to obtain white solid CJHB761, yield 84%. EI-MS / FAB, m / e: 530.76, calculated: 530.20.
[0107] With reference to the above-mentioned synthetic method, the following compounds were prepared:
[0108]
[0109]
[0110]
[0111]
[0112]
[0113]
[0114]
[0115]
Embodiment 3
[0117] The preparation method of compound CJHB775 comprises the steps:
[0118] The first step: preparation of compound Int.-5
[0119]
[0120] Under the protection of nitrogen, 50.0mmol of A1 was dissolved in 120mL of dry THF, cooled to -100°C, 24mL of 2.5M n-butyllithium n-hexane solution was added dropwise, stirred for 30 minutes, and 75.0mmol of triisoborate was added dropwise. Butyl ester, stir for 30 minutes, heat up to -70°C for 1 hour, add dropwise 50 mL of 2N dilute hydrochloric acid aqueous solution, stir for 30 minutes, separate the organic phase, extract the aqueous phase with ethyl acetate, collect the organic phase, dry, and filter , the filtrate was concentrated and dried under reduced pressure, dispersed by adding petroleum ether, and filtered to obtain Int.-5 with a yield of 85%.
[0121] The second step: the preparation of compound CJHB775
[0122]
[0123] Mix 12.0mmol of intermediate Int.-5 with 60mL of toluene, and add 10.0mmol of 2-chloro-4,6-dip...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com