Sulfur-containing amino alcohol Schiff base copper complex, and preparation method and application thereof
A technology of thioamino alcohol and Schiff base copper, used in copper organic compounds, drug combinations, antitumor drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
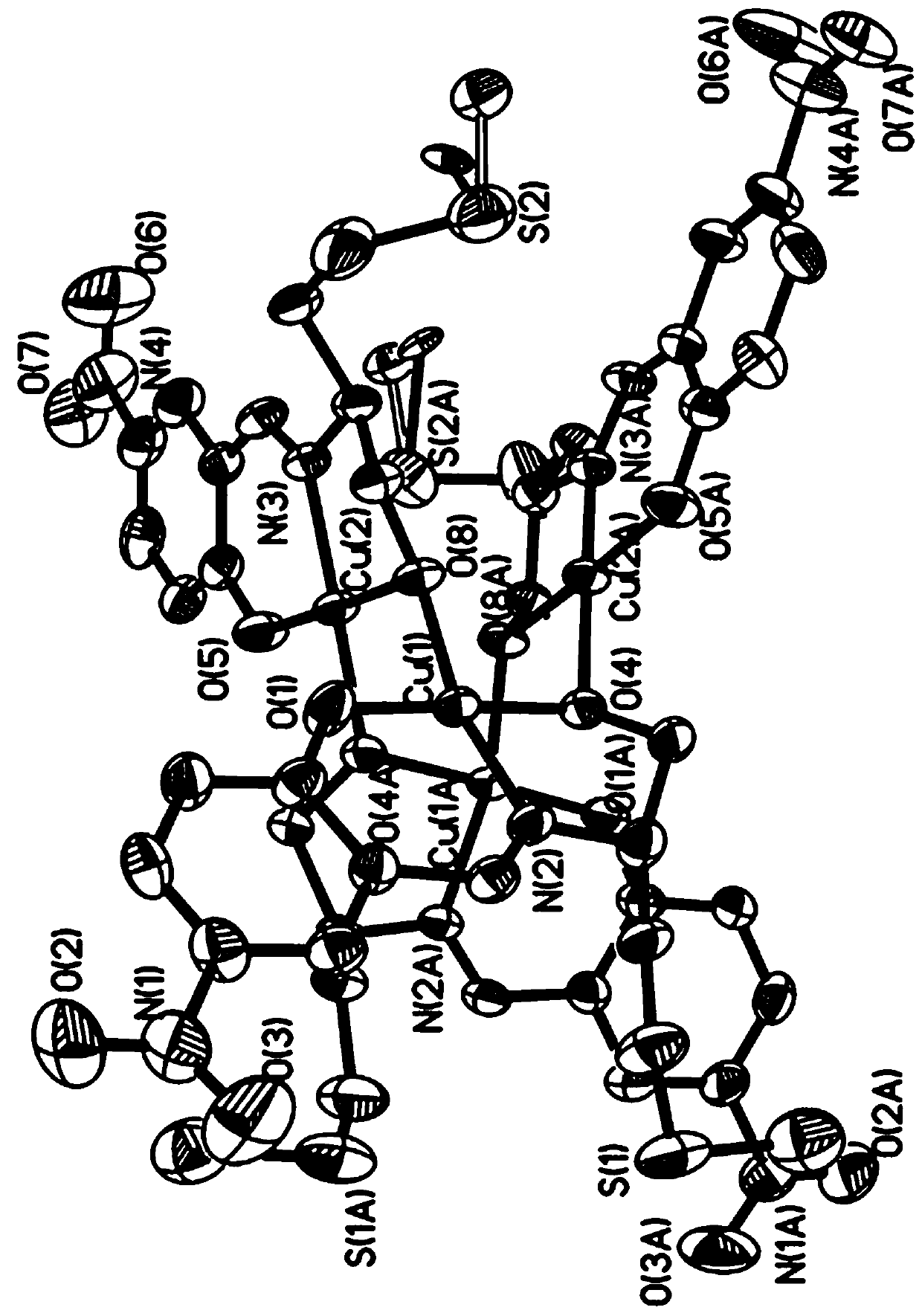
[0025] Example 1 [Cu 4 (C 12 H 14 N 2 O 4 S) 4 ] Preparation of single crystals
[0026]Dissolve L-methioninol with anhydrous methanol to prepare L-methioninol anhydrous methanol solution with a concentration of 0.1 mmol / mL; dissolve KOH solid with anhydrous methanol and prepare a KOH anhydrous solution with a concentration of 0.05 mmol / mL methanol solution. In the reactor of 25mL, add L-methioninol anhydrous methanol solution 2mL (0.2mmol), 5-nitrosalicylaldehyde 0.0334g (0.2mmol), KOH anhydrous methanol solution 6mL (0.3mmol) and anhydrous Methanol 8mL, stir the solution magnetically at room temperature and stir into a vortex, and the solution is egg-yellow. After stirring for 1 h, Cu(NO) was added to the solution. 3 ) 2 ·3H 2 O (0.1216 g, 0.5 mmol) and 6 mL of anhydrous methanol were stirred for 1 h to obtain a green liquid with a small amount of turbidity. After stopping stirring, tighten the lid of the reaction kettle and place it in a constant temperature blas...
Embodiment 2
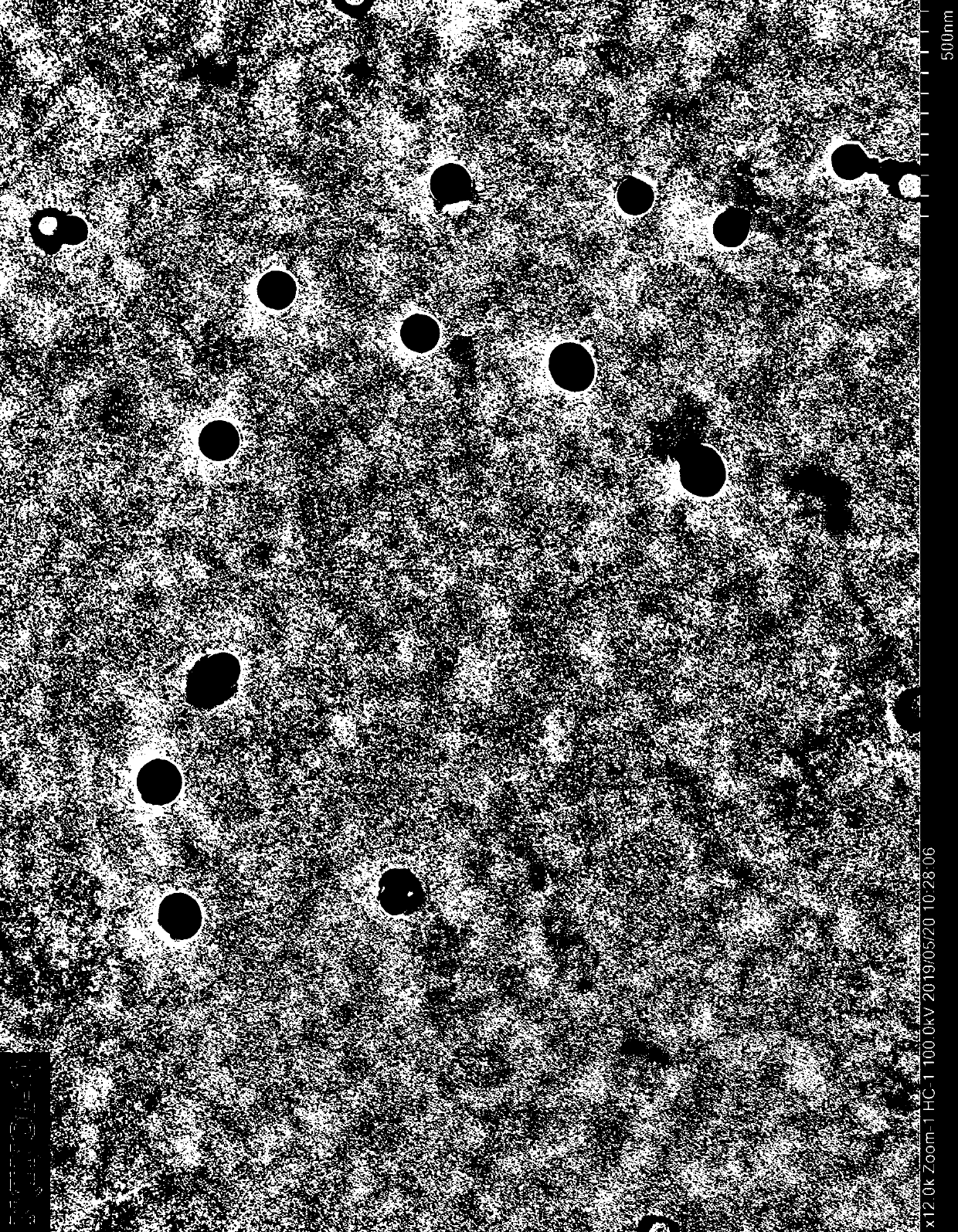
[0027] Example 2 [Cu 4 (C 12 H 14 N 2 O 4 S) 4 ] Preparation of nanoparticles
[0028] will [Cu 4 (C 12 H 14 N 2 O 4 S) 4 ] The single crystal was first dissolved in DMSO, and then slowly added with ethanol to form a solution with a mixed solvent of DMSO-ethanol (volume ratio 1:1), with a final concentration of 2.0 mmol.L -1 , detected by transmission electron microscope, and observed [Cu 4 (C 12 H 14 N 2 O 4 S) 4 ] spherical nanoparticles N(TNCu-1), such as figure 2 shown.
Embodiment 3
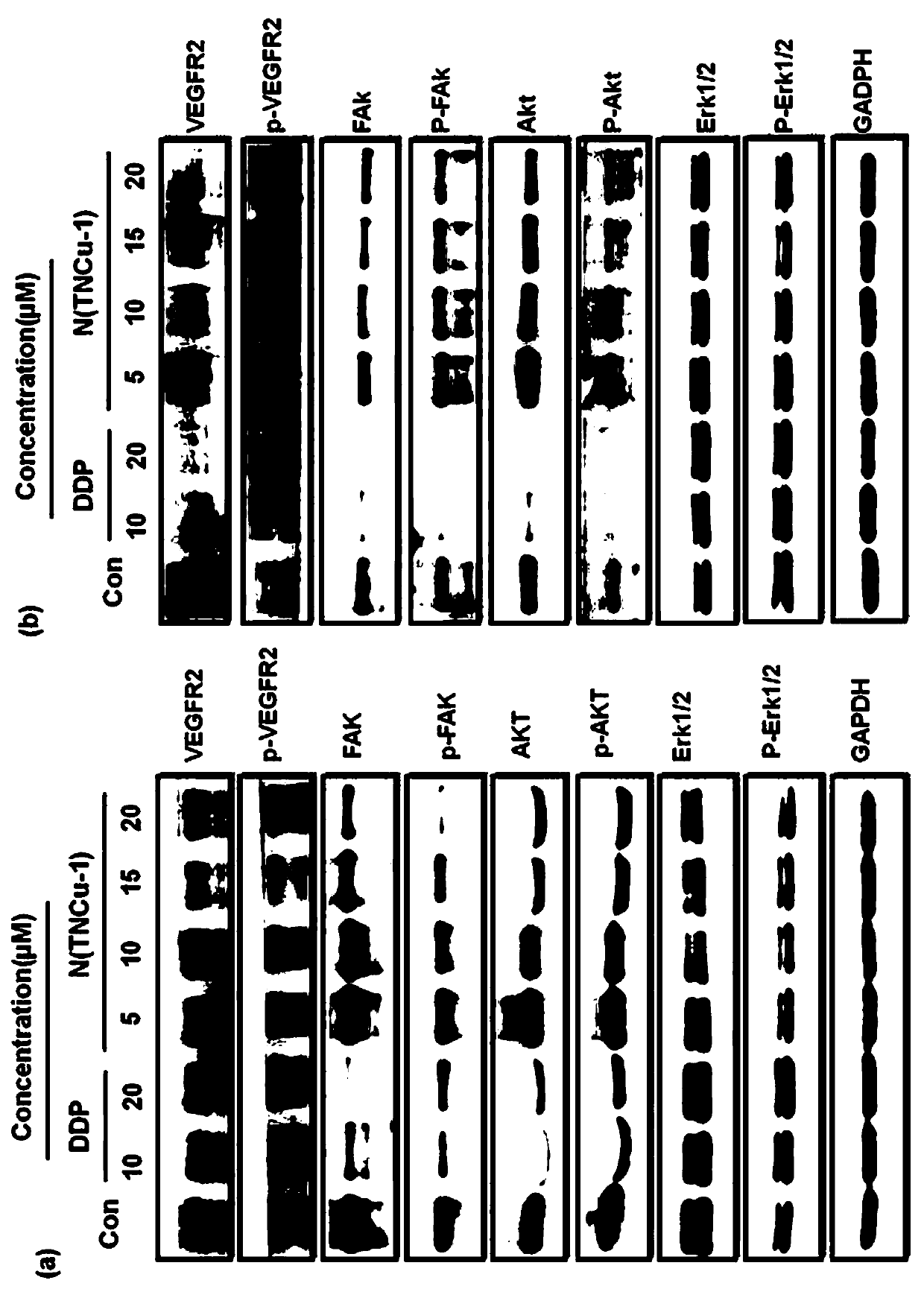
[0029] Example 3 [Cu 4 (C 12 H 14 N 2 O 4 S) 4 ] Nanoparticles in vitro cytotoxicity assay
[0030] MTT method: Take tumor cells in logarithmic growth phase, adjust the concentration of viable cells to 5 × 10 4 100 μL per well was added to a 96-well culture plate. After culturing in the incubator for 18 hours, 100 μL of different concentrations of test samples diluted with serum-free culture were added respectively. The sample addition group was set to 6 replicate wells for each concentration. , at the same time as a negative control, placed at 37 ° C, 5% CO 2 Incubate for 48h, then add MTT (5mg / mL) 20μL / well, after 4h, use a microsyringe to gently aspirate the clear night, add dimethyl sulfoxide (DMSO) 150μL / well, shake for about 10min, use a microplate reader at 490nm wavelength OD value was determined. Calculate the cell survival inhibition rate and calculate its half inhibitory concentration IC through the software 50 .
[0031] Inhibition rate = (OD 阴性组平均值 -OD ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com