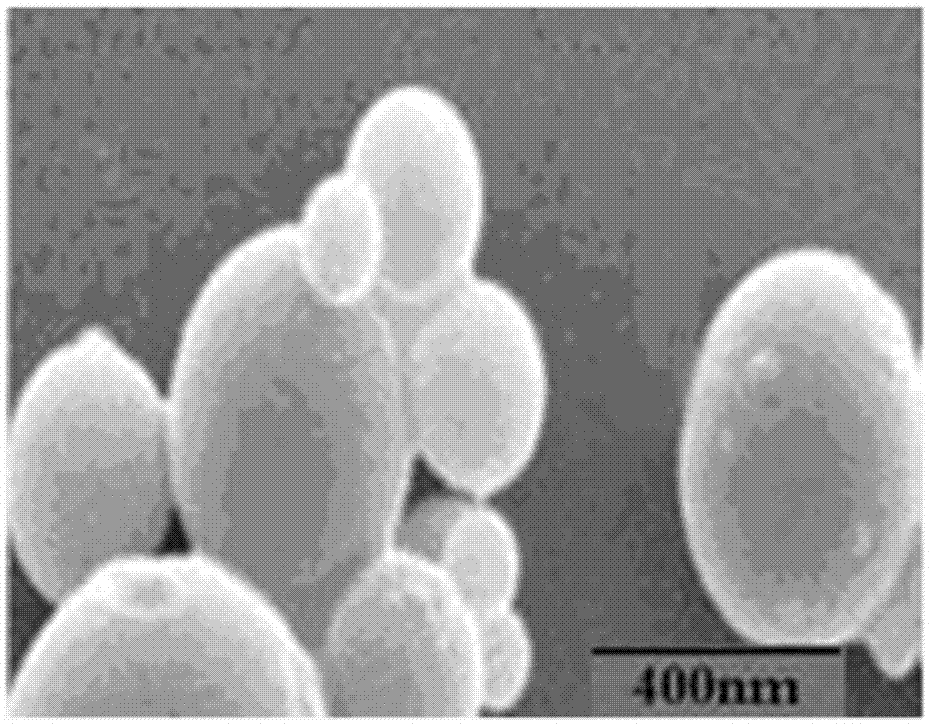
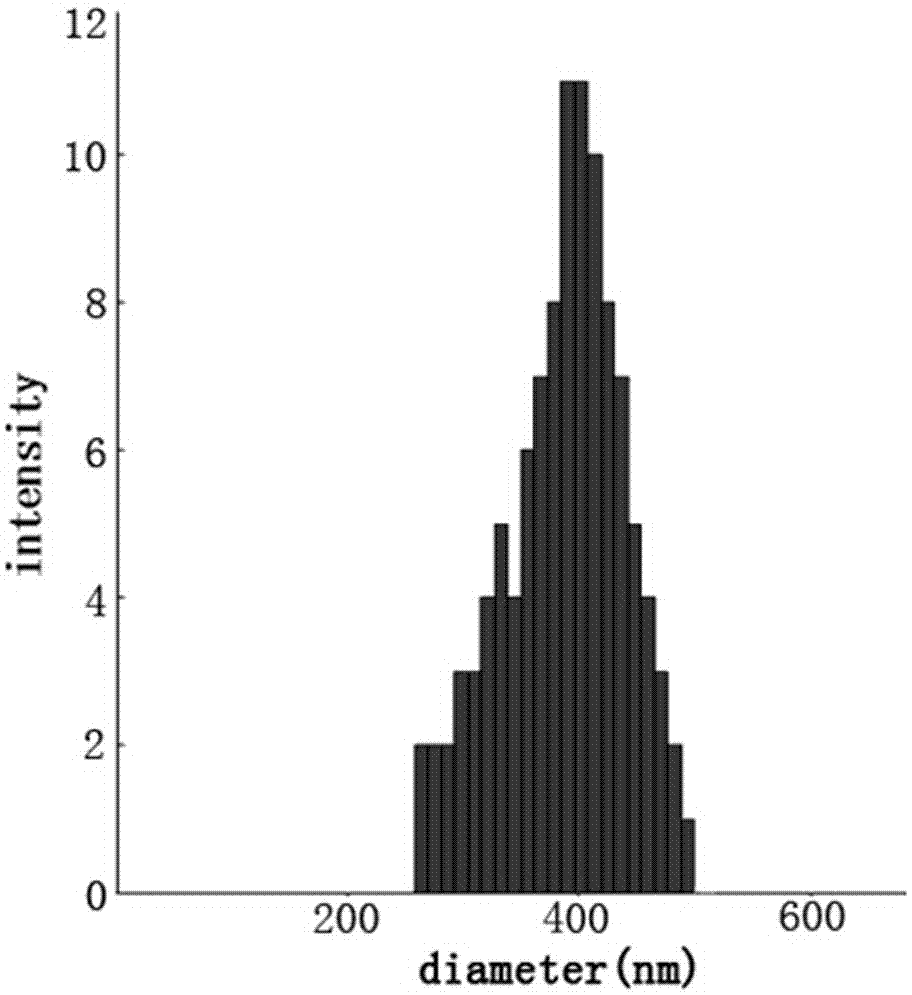
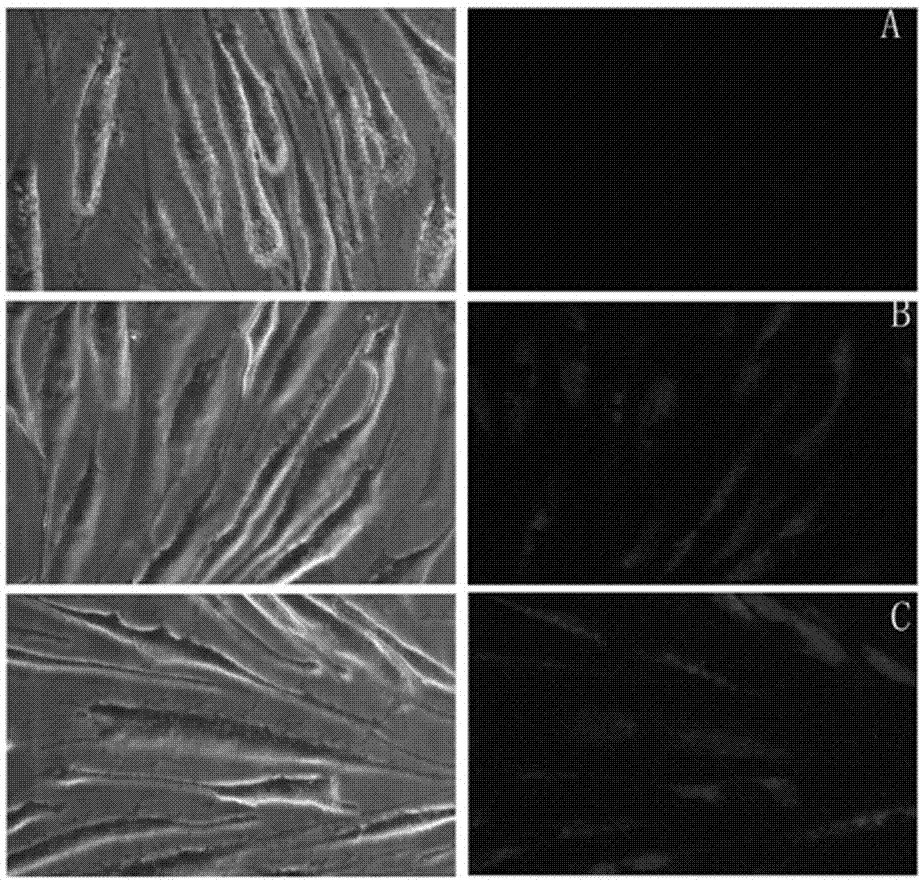
A kind of chitosan carries siRNA coating vascular stent and preparation method thereof
A vascular stent, chitosan technology, applied in the field of vascular stent, can solve the problems of poor water solubility, poor targeting, low transfection efficiency, etc., to prevent thrombosis and regeneration, induce cell apoptosis, and treat cell proliferation diseases. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036]A kind of preparation method that chitosan carries siRNA coating vascular stent, comprises the following steps:
[0037] (1) Construction of TF-siRNA target sequence: TF-mRNA 167-186bp sequence, the sequence is 5-GCG CTT CAG GCACTA CAA AT-3, labeled with fluorescein to obtain TF-siRNA tissue factor;
[0038] (2) Preparation of hydroxybutyl chitosan-carried tissue factor siRNA nanoparticles:
[0039] (a) Take the layered sample of hydroxybutyl chitosan, tear it into thin slices with clean tweezers, put it in a flat glass petri dish, put it under the ultraviolet lamp and irradiate the front and back sides for 50-80 minutes, then dissolve it in acetic acid solution and filter Sterilize to get hydroxybutyl chitosan acetic acid solution;
[0040] (b) Take sodium tripolyphosphate (TPP), add water to dissolve, stir evenly, filter and sterilize to obtain TPP aqueous solution;
[0041] (c) Take the TF-siRNA tissue factor obtained in step (1) and add it to DEPC (diethylpyrocarbo...
Embodiment 1
[0051] A preparation method of hydroxybutyl chitosan carrying tissue factor siRNA coating vascular stent, comprising the following steps:
[0052] (1) Construction of the TF-siRNA target sequence: First, search for the full-length 2104bp mRNA sequence of coagulation factor III (TF) in NCBI (M16553.1, GI: 339503); the TF-siRNA target sequence screened in this experiment was selected for The 167-186bp sequence of TF-mRNA (5-GCG CTT CAG GCA CTA CAA AT-3) was handed over to the company to design its silencing sequence and labeled with FAM fluorescence to obtain TF-siRNA tissue factor.
[0053] (2) Preparation of hydroxybutyl chitosan-carried tissue factor siRNA nanoparticles:
[0054] (a) Accurately weigh 3 mg of dry hydroxybutyl chitosan sheet-like sample, tear it into thin slices with clean tweezers, lay it flat on a glass petri dish, place it under a UV lamp for 60 minutes on the front and back sides, and then dissolve it in 3 mL Acetic acid solution (concentration of acetic a...
Embodiment 2
[0062] A kind of preparation method that chitosan carries siRNA coating vascular stent, comprises the following steps:
[0063] (1) Construction of the TF-siRNA target sequence: First, search for the full-length 2104bp mRNA sequence of coagulation factor III (TF) in NCBI (M16553.1, GI: 339503); the TF-siRNA target sequence screened in this experiment was selected for The 167-186bp sequence of TF-mRNA (5-GCG CTT CAG GCA CTA CAA AT-3) was submitted to the company to design its silencing sequence and labeled with fluorescein to obtain TF-siRNA tissue factor.
[0064] (2) Preparation of hydroxybutyl chitosan-carried tissue factor siRNA nanoparticles:
[0065] (a) Accurately weigh 1.5 mg of dry hydroxybutyl chitosan sheet-like sample, tear it into thin slices with clean tweezers, lay it flat on a glass petri dish, place it under a UV lamp for 50 minutes on the front and back sides, and then dissolve it in 3mL acetic acid solution (the concentration of acetic acid is 0.2M, the pH v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com