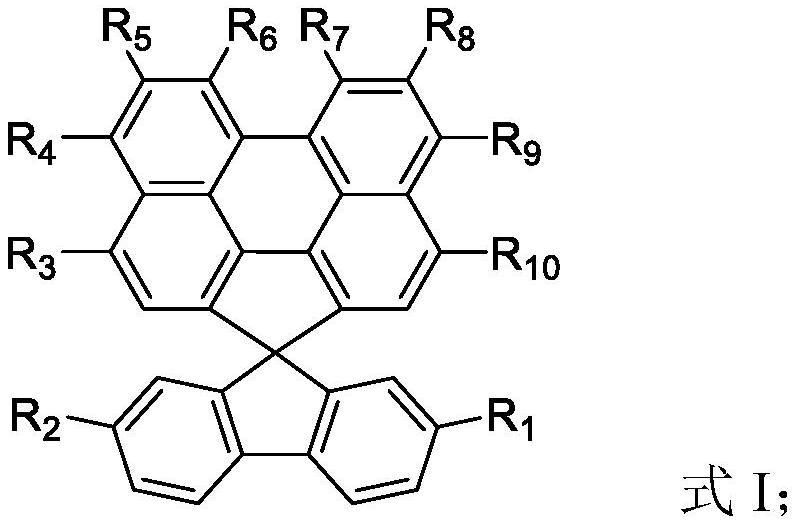
Compounds, organic electroluminescent materials, organic electroluminescent devices, electronic devices
A technology of organic light-emitting layers and compounds, applied in the direction of light-emitting materials, electric solid devices, silicon organic compounds, etc., can solve the problems of difficult molecular design, poor color purity, and aggravated molecular fluorescence quenching, so as to increase the internal electron density and stabilize properties, increased steric hindrance, and increased density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
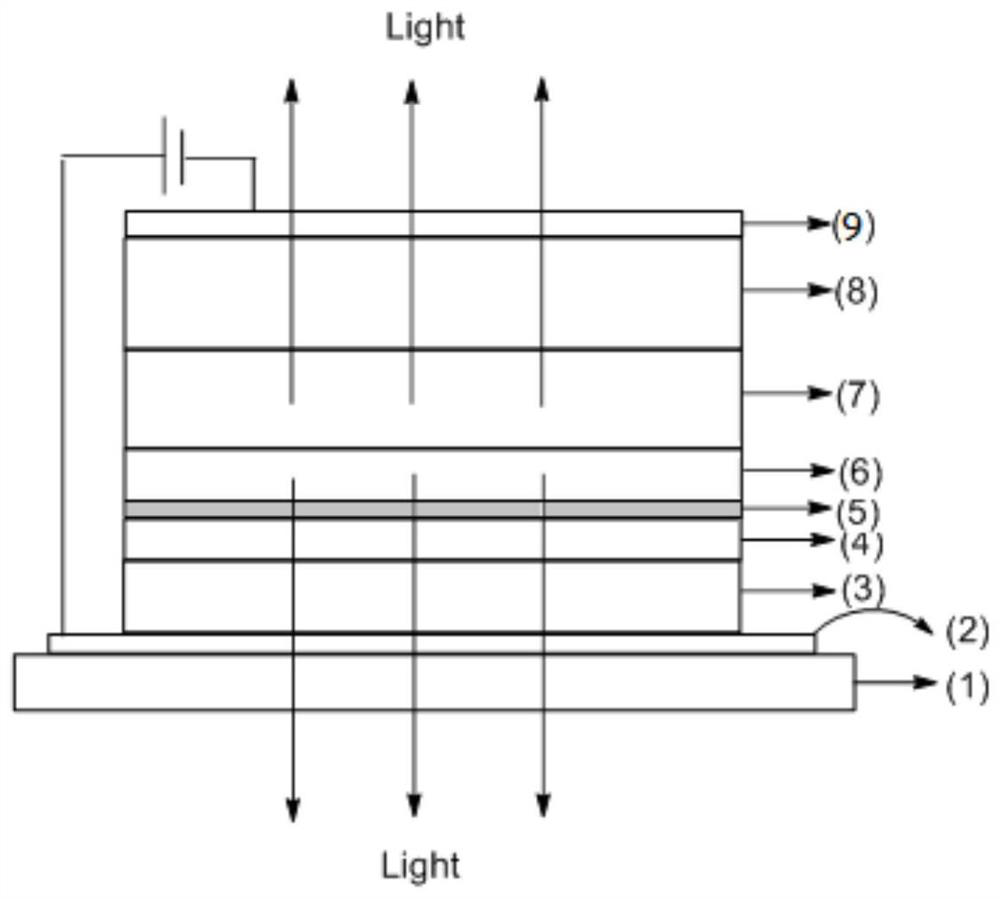
[0070] As the preparation method of the organic electroluminescent device of the present invention, the following preparation methods can be listed, but not limited thereto, and those skilled in the art can make various changes according to the technical common knowledge in the field. The aforementioned preparation method comprises the following steps:
[0071] Cleaning process: use cleaning agent, deionized water, organic solvent, etc. to clean the glass substrate with ITO;
[0072] The step of forming a hole injection layer: a hole injection layer forming material containing the metal complex of the present invention is vapor-deposited on the aforementioned anode layer by vacuum evaporation, thereby forming holes containing the metal complex of the present invention on the aforementioned substrate. hole injection layer;
[0073] The process of forming a hole transport layer: forming a hole transport layer on the aforementioned hole injection layer by vacuum evaporation;
...
Embodiment 1
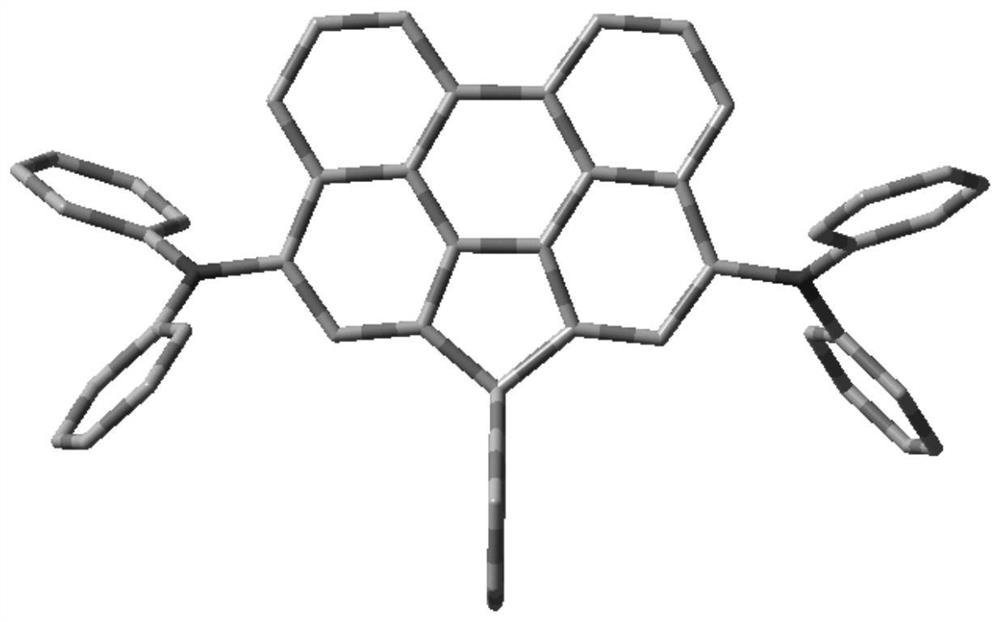
[0088] Preparation of compound SLC-B203:
[0089] The preparation method of compound SLC-B203, comprises the steps:
[0090] The first step: preparation of compound Int-1
[0091]
[0092] 10.0g (42.9mmol) of 2-bromobiphenyl (CAS: 2052-07-5) was dissolved in 100ml of dry THF, under the protection of nitrogen, the temperature was lowered to -78°C with liquid nitrogen, and 19ml of 2.5M n-Butyllithium n-hexane solution, stirred and reacted for 30 minutes, slowly added dropwise 13.0g (42.0mmol) of methyl 1-perylenecarboxylate (CAS: 2097797-89-0) dissolved in anhydrous THF, stirred and reacted for 1 hour Afterwards, rise to room temperature naturally, stir and react for 24 hours, add dropwise the saturated aqueous ammonium chloride solution of 100ml to quench the reaction, separate the organic phase, extract the aqueous phase with dichloromethane, and dry the organic phase with anhydrous sodium sulfate, filter, and reduce Concentrate to dryness under pressure to obtain a yello...
Embodiment 2
[0101] Preparation of Compound SLC-B246:
[0102]
[0103] Referring to the preparation method of the third step of Example 1, replace 4,4'-dimethyldiphenylamine in the third step of Example 1 with N-(4-isopropylphenyl) dibenzo[b,d ]furan-4-amine (CAS: 1252914-26-3) was obtained as an orange solid with a yield of 59%. 1 H-NMR (δ, CDCl 3 ): 8.164(2H,d), 8.003(2H,d), 7.836(2H,d), 7.558~7.530(4H,m), 7.501~7.476(8H,m), 7.322~7.253(8H,m), 7.146~7.085(8H,m), 6.862~6.841(4H,m), 2.413(2H,t), 1.214(6H,s)1.195(6H,s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com