Benzophenanthrene derivative and application thereof
A derivative, triphenylene technology, applied in triphenylene derivatives and their application fields, can solve the problems of reducing the quantum yield of the blue light system, poor color purity, low luminescence quantum efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
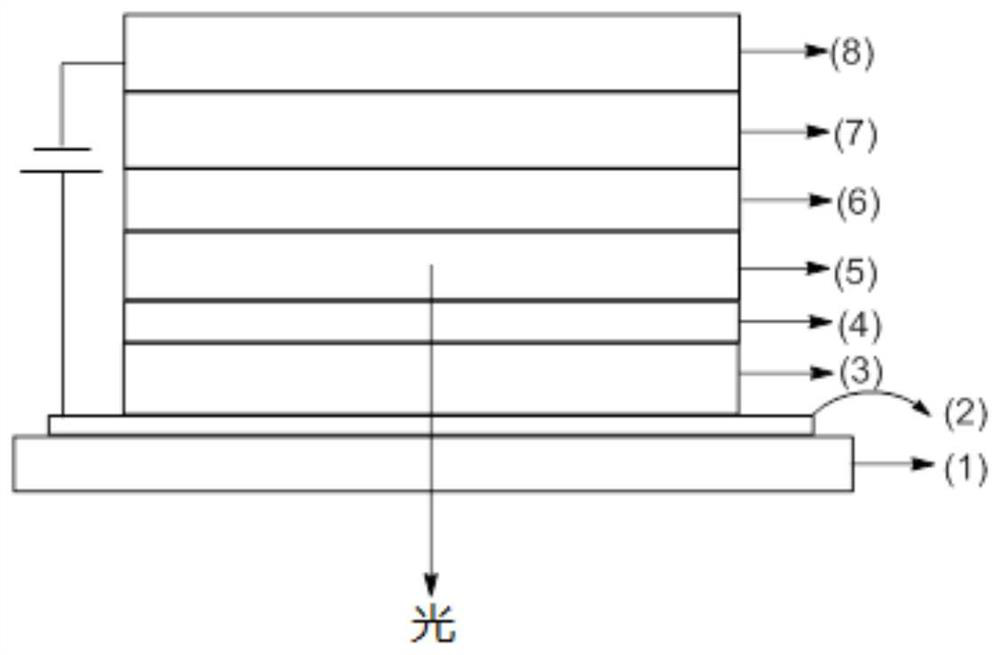
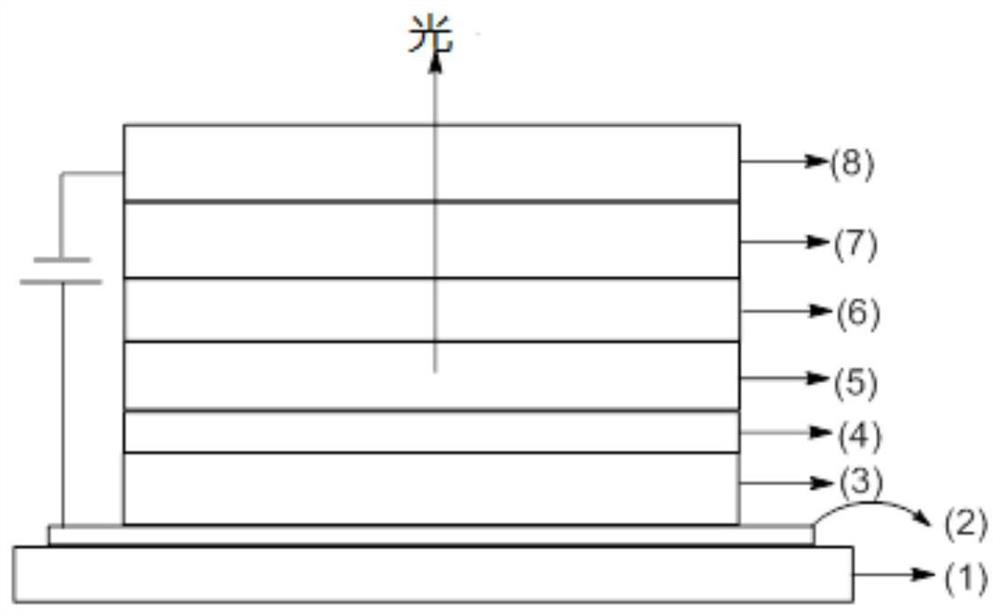
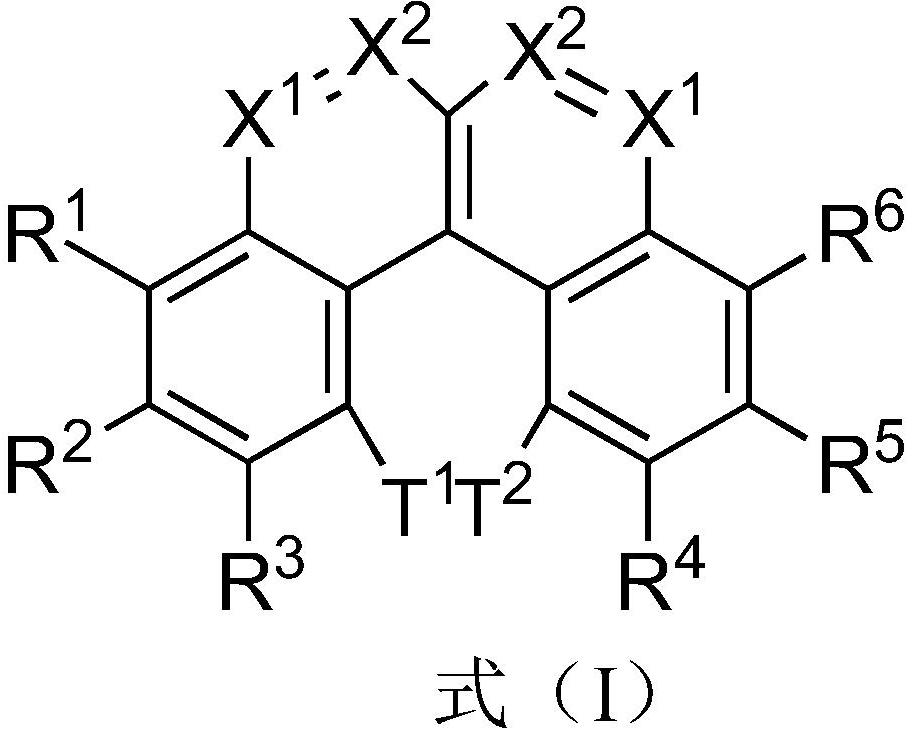
Image
Examples
Embodiment 1
[0071] The preparation method of compound CJHB271 comprises the following steps:
[0072] Step 1: Preparation of Compound Int.-1
[0073]
[0074] Under the protection of nitrogen, 24.5g (127.0mmol) of p-chlorobromobenzene was dissolved in 300mL of dry tetrahydrofuran, cooled to -78°C with liquid nitrogen, and 56.0mL of 2.5M n-butyllithium n-hexane solution was slowly added dropwise, and stirred React for 1 hour, add dropwise a solution of 19.5g (140.0mmol) of p-chlorobenzonitrile dissolved in 50mL of dry tetrahydrofuran, rise to room temperature and stir for 2 hours, add 50mL of saturated ammonium chloride aqueous solution, stir for 30 minutes, separate the organic phase, the water phase was extracted with ethyl acetate, the organic phase was collected and dried, filtered, the filtrate was concentrated to dryness under reduced pressure, separated and purified with a silica gel column to obtain 27.7g of white solid, the yield was 87%.
[0075] The second step: the preparat...
Embodiment 2
[0090] The preparation method of compound CJHB387, comprises the steps:
[0091] The first step: preparation of compound Int.-5
[0092]
[0093] Under the protection of nitrogen, 12.8g (50.0mmol) of 2,7-dimethoxyxanth-9-one was dissolved in 200mL of dichloromethane, cooled to 0°C in an ice-water bath, and 20.0g of tetrabromide carbon and 12.5g of triisopropyl phosphite, stirred and reacted for 30 minutes, raised to room temperature and reacted for 1 hour, added dropwise 50mL of 1N dilute hydrochloric acid aqueous solution, stirred for 30 minutes, separated the organic phase, extracted the aqueous phase with dichloromethane, The organic phase was collected, dried, filtered, and the filtrate was concentrated to dryness under reduced pressure, separated and purified by silica gel column to obtain Int.-5 as a white solid with a yield of 94%.
[0094] The second step: the preparation of compound Int.-6
[0095]
[0096] Referring to the preparation method in the third step...
Embodiment 3
[0113] Preparation of compounds CJHB270~CJHB324, CJHB386, CJHB388~CJHB403:
[0114] Referring to the preparation method of Examples 1-2, only the diphenylamine in the fifth step in Example 1 or the 4,4'-dimethyldiphenylamine in the sixth step in Example 2 were replaced with different swollen amines, and other experimental parameters Make routine adjustments.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com