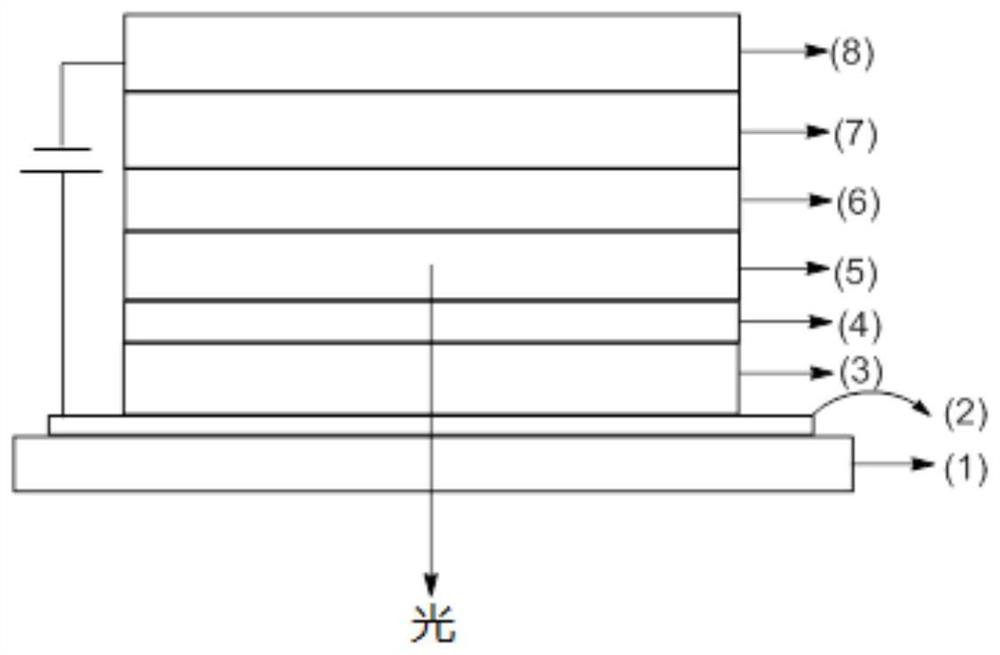
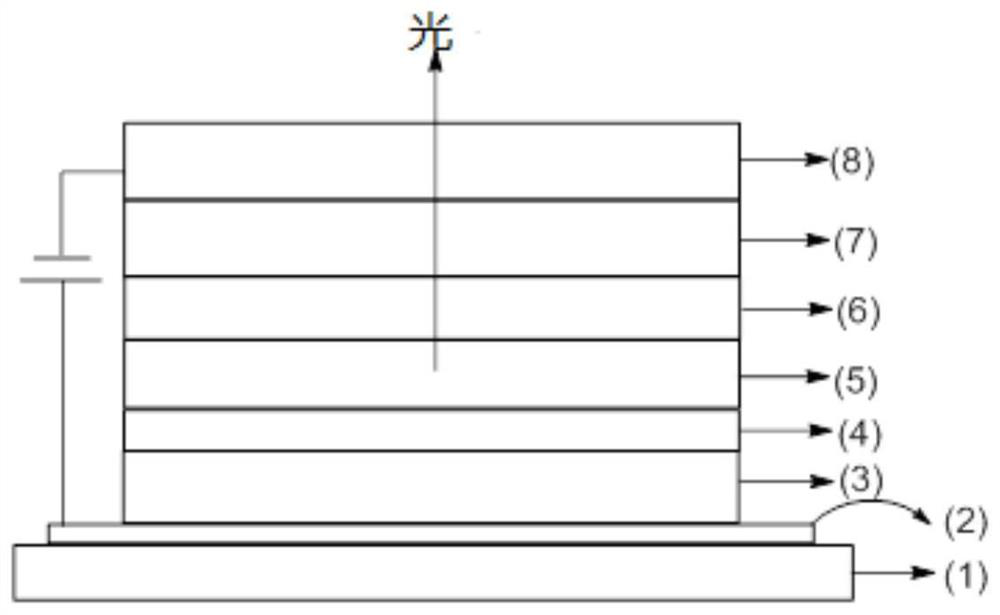
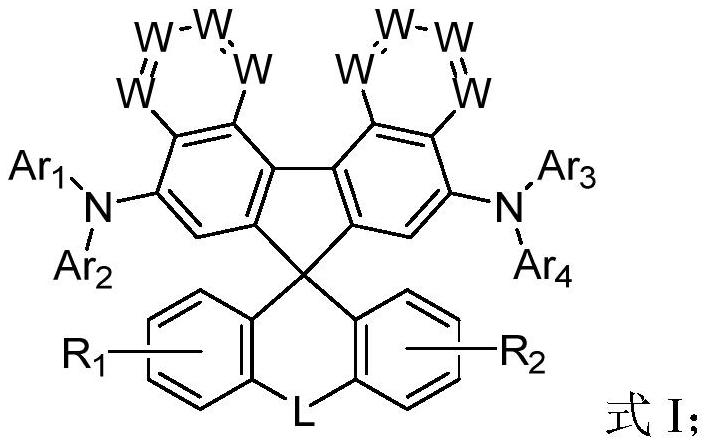
Spirofluorene derivative and application thereof
A derivative, spirofluorene technology, applied in the field of spirofluorene derivatives and its applications, can solve the problems of molecular design difficulties, aggravated molecular fluorescence quenching, poor color purity, etc., to increase internal electron density and stability, and improve dissolution Sexuality, the effect of lowering the temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] The preparation method of compound SLC-B441, comprises the steps:
[0082] The first step: preparation of compound Int-1
[0083]
[0084] Under nitrogen protection, 7.65g (37.5mmol) of 2-bromo-3-methoxyphenol was dissolved in 120ml of dry dichloromethane, and 5.75ml of triethylamine was added, cooled to -78°C with liquid nitrogen, and slowly Add 6.94ml of trifluoromethanesulfonic anhydride dropwise, stir for half an hour, raise the temperature to 0°C and stir for 1 hour, add 50ml of saturated ammonium chloride aqueous solution, stir for 30 minutes, separate the organic phase, and use dichloromethane for the aqueous phase After extraction, the organic phase was collected and dried, filtered, and the filtrate was concentrated to dryness under reduced pressure to obtain 11.9 g of yellow oil.
[0085] Add 10.35 g (75.0 mmol) of anhydrous potassium carbonate, 12.5 g (41.2 mmol) of (2-((isopropylsilyl) ethynyl) phenyl) boronic acid, and then add 526.5 mg (0.75 mmol) of P...
Embodiment 2
[0119] Preparation of compound SLC-B422:
[0120]
[0121] With reference to the preparation method of Example 1, only the 4-phenanthrene boronic acid in the fourth step in Example 1 is replaced by 1-phenanthrene boronic acid to prepare intermediate Int-11, and the intermediate Int-11 in the eleventh step in Example 1 is 10 was replaced by Int-11 to prepare compound SLC-B422, yellow solid, MS (MALDI-TOF): m / z 1074.5744 [M + ].
Embodiment 3
[0123] Preparation of compounds SLC-B421, SLC-B423~SLC-B428, SLC-B435~SLC-B440, SLC-B442~SLC-B444:
[0124] With reference to the preparation method of Example 1 and Example 2, the bis(4-(tert-butyl)phenyl)amine in the eleventh step of Example 1 was replaced by the corresponding substituted arylamine to prepare the target compound SLC-B421, SLC -B423~SLC-B428, SLC-B435~SLC-B440, SLC-B442~SLC-B444.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com