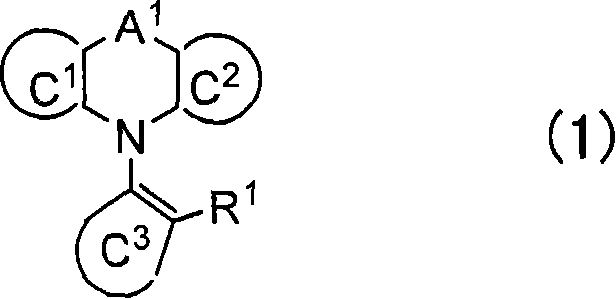
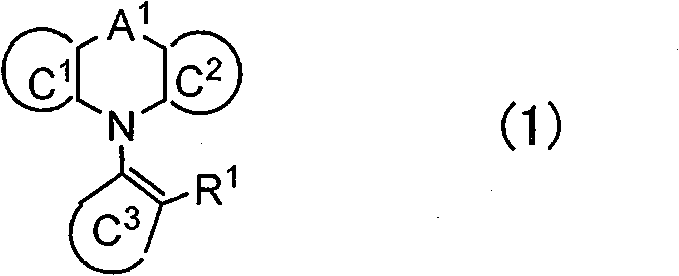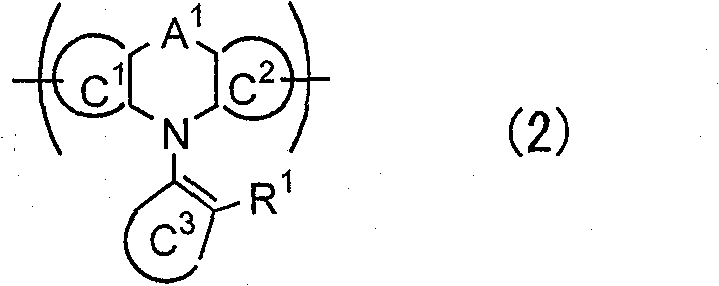Polymer and light-emitting polymer element using the same
A technology of polymer compounds and luminescent materials, which is applied in the field of polymer light-emitting elements, and can solve problems such as long emission wavelengths and insufficient characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0423] Synthesis of Compound B and Compound B-1
[0424] (Synthesis of Compound A)
[0425]
[0426] Install a reflux tube on the three-necked flask. Under a nitrogen atmosphere, 10.0 g of phenoxazine, 15.2 g of 1-bromo-4-tert-butyl 2,6-dimethylbenzene, 21.9 g of sodium tert-butoxide, and 345 ml of toluene were added and stirred, and then tridibenzylidene was added. 0.25 g of acetone dipalladium, and 0.13 g of tert-butylphosphine tetrafluoroborate. Stir at reflux for 9 hours and cool to room temperature. The reaction solution was filtered through a glass filter precoated with alumina, the resulting solution was washed with 3.5% hydrochloric acid, and the toluene solution was concentrated. To the obtained solid, 5 ml of toluene and 50 ml of isopropanol were added, heated, stirred for 1 hour, and then cooled to room temperature. The generated precipitate was filtered and washed with isopropanol to obtain 8.3 g of Compound A as a pale yellow solid.
[0427] 1 H-NMR (CDC...
Synthetic example 1
[0439] Synthesis of Compound D
[0440] (Synthesis of Compound C)
[0441]
[0442]Install a mechanical stirrer and a condenser on a 3L four-neck round bottom flask. The inside of the reaction vessel was replaced with nitrogen, 1.10 g of palladium(II) acetate, 1.51 g of tris(o-tolyl)phosphine, and 368 ml of toluene were added, and the mixture was stirred at room temperature for 30 minutes. 143 g of phenoxazine, 97.1 g of sodium tert-amyloxide, and 800 ml of toluene were added and stirred, 133.4 ml of 1-bromo-4-butylbenzene was dissolved in 60 ml of toluene, and added dropwise to the reaction vessel using a dropping funnel. Stir at 105°C for 5 hours, then cool to room temperature. Filtration was performed through a glass filter pre-coated with 2 cm of alumina, and the resulting solution was neutralized with 3.5% hydrochloric acid. The toluene solution was concentrated, 30 ml of toluene was added, and the mixture was stirred at 75° C. for 30 minutes, and then 700 ml of is...
Synthetic example 2
[0450] Synthesis of Compound F
[0451] (Synthesis of Compound E)
[0452]
[0453] Install a mechanical stirrer and a condenser on a 3L three-necked round-bottomed flask for nitrogen replacement. Then, 86.5 g of 2,7-dibromo-9-fluorenone and 500 g of phenol dissolved by heating in a furnace were added. The temperature was raised to 105°C while stirring, and cooled to 65°C when 2,7-dibromo-9-fluorenone was completely dissolved. 1.98 g of 3-mercaptopropane-1-sulfonic acid was weighed in a spherical box, and it was slowly added while being careful not to raise the temperature in the reaction system. After adding the catalyst, the mixture was stirred at 65° C. for 21 hours, 722 ml of ethanol was added, and heated to dissolve. It was then cooled to 45°C, poured into 7.6L of ion-exchanged water heated to 65°C, and then stirred for 2 hours. The precipitated orange precipitate was filtered, washed with water, and left to dry overnight. The obtained orange solid was transferre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com