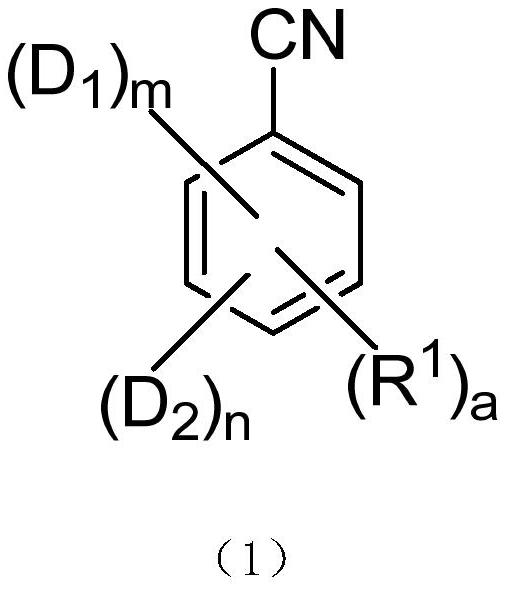
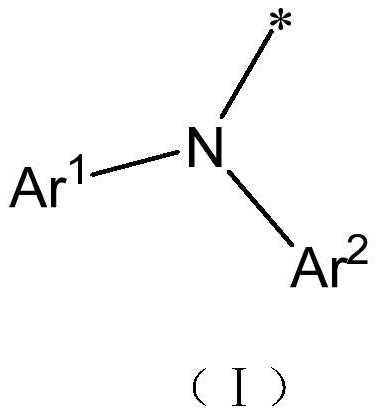
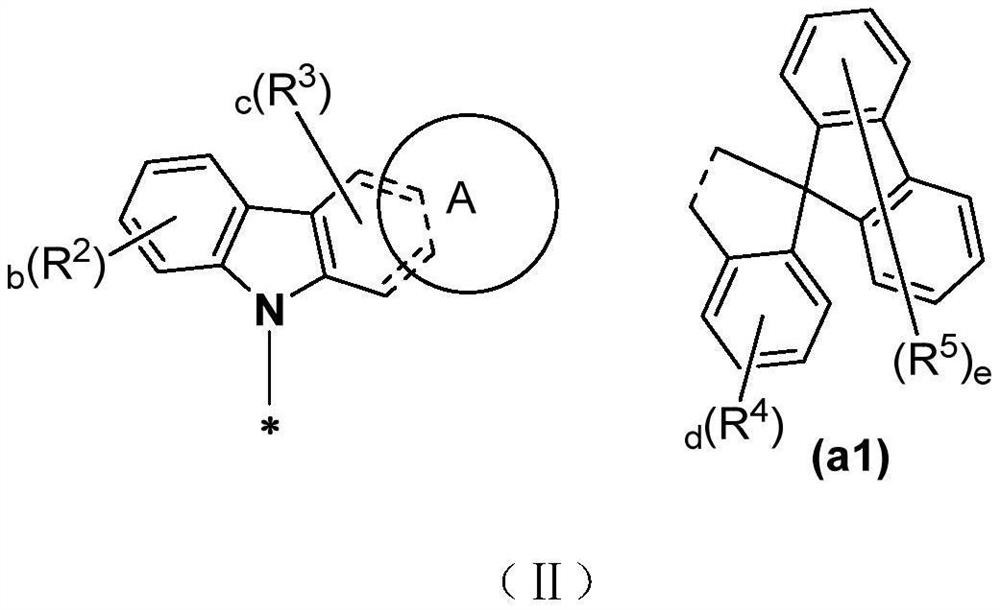
Thermally activated delayed fluorescent material, organic electroluminescent device and application of thermally activated delayed fluorescent material
A kind of compound, the technology of compound of general formula, applied in the field of organic electron light-emitting device
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0080] Synthesis Example 1: Synthesis of S1
[0081]
[0082] Synthesis of Intermediate S1-1:
[0083]Add carbazolospirofluorene (14.54g, 35.85mmol), 2,6-dibromo-4-fluorobenzonitrile (10g, 35.85mmol), cesium carbonate (23.36g, 71.71mmol) into a 500ml single-necked bottle at room temperature, N,N-Dimethylformamide (200ml) was reacted overnight at 120°C under nitrogen protection. Stop heating, after cooling to room temperature, add 500ml of water and stir for 10min, a large amount of white solid precipitates, filter with suction, wash the filter cake with ethanol for 2h, cool down and filter with suction to obtain 18g of white solid product, yield 75.6%. Molecular ion mass determined by mass spectrometry: 664.34 (theoretical value: 664.40).
[0084] Synthesis of Compound S1:
[0085] At room temperature, S1-1 (5g, 7.53mmol), carbazole (2.52g, 12.05mmol), Pd 2 (dba) 3 (0.69g, 0.75mmol), P(t-Bu) 3 (0.30g, 1.51mmol), sodium tert-butoxide (2.17g, 22.58mmol), and xylene (...
Synthetic example 2
[0086] Synthesis Example 2: Synthesis of S2
[0087]
[0088] Synthesis of compound S2:
[0089] At room temperature, S1-1 (5g, 7.53mmol), 3,6-dimethylcarbazole (2.94g, 15.05mmol), Pd 2 (dba) 3 (0.69g, 0.75mmol), P(t-Bu) 3 (0.30g, 1.51mmol), sodium tert-butoxide (2.17g, 22.58mmol), and xylene (50ml) were added to a 100ml single-necked bottle, pumped with nitrogen three times, and heated to 130°C to react overnight. The reaction solution was lowered to room temperature, filtered, and the filtrate was concentrated by mixing silica gel. Column chromatography (PE: EA = 100:1) gave 5 g of crude product. Toluene / ethanol recrystallization gave 4 g of white solid, yield 59.5%. Molecular ion mass determined by mass spectrometry: 864.44 (theoretical value: 864.33).
Synthetic example 3
[0090] Synthesis Example 3: Synthesis of S4
[0091]
[0092] Synthesis of compound S4:
[0093] At room temperature, S1-1 (5g, 7.53mmol), 3-isopropylcarbazole (3.78g, 15.05mmol), Pd 2 (dba) 3 (0.69g, 0.75mmol), P(t-Bu) 3 (0.30g, 1.51mmol), sodium tert-butoxide (2.17g, 22.58mmol), and xylene (50ml) were added to a 100ml single-necked bottle, pumped with nitrogen three times, and heated to 130°C to react overnight. The reaction solution was lowered to room temperature, filtered, the filtrate was concentrated by mixing silica gel, column chromatography (PE:EA=100:1) obtained 5.2 g of crude product, recrystallized from toluene / ethanol to obtain 4.5 g of white solid, yield 46.7%. Molecular ion mass determined by mass spectrometry: 920.41 (theoretical value: 920.39).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com