Preparation and application of a thermally activated delayed fluorescence OLED based on a seven-membered ring diimide acceptor
A heat-activated delay and cyclic imide technology, which is applied in the fields of luminescent materials, semiconductor/solid-state device manufacturing, organic chemistry, etc., can solve the problems of restricting the development of high-efficiency delayed fluorescent materials, low device efficiency, and single types, etc., and achieve good results. The effect of industrialization prospect, good performance and good application effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of Intermediate B1
[0034]
[0035] A magnetic stirring bar was placed in a 250mL there-necked flask, p-bromobenzoic acid (10g, 50mmol), ruthenium trichloride (II) (518.6mg, 2.5mmol), 1,8-diazabicyclo[5.4.0 ] Undec-7-ene (7.2 mL, 50 mmol), replaced oxygen for more than three times, added 60 mL of ethylene glycol dimethyl ether, heated to 110° C. and reacted for 30 hours. After the reaction was completed and cooled to room temperature, potassium carbonate (20.7 g, 150 mmol), methyl iodide (9.5 mL, 150 mmol) were added, and after stirring at room temperature for 4 hours, the solution was filtered under reduced pressure, and the solvent and low boilers were evaporated in vacuo. The crude product was filtered through a column. Purified by chromatography, the eluent was petroleum ether:ethyl acetate=10:1. The target intermediate B1 was obtained in the form of 5.56 g of white solids, and the yield was 52%.
[0036] High-resolution mass spectrometry, ESI sour...
Embodiment 2
[0052] Preparation of Intermediate D1
[0053]
[0054] A magnetic stirring bar was placed in a 250mL there-necked flask, p-bromobenzoic acid (10g, 50mmol), benzoic acid (1.525g, 12.5mmol), dichloro (p-methylcumene) ruthenium (II) dimer were added (736mg, 1.25mmol), copper oxide (1.5g, 18.75mmol), 1,8-diazabicyclo[5.4.0]undec-7-ene (9.0mL, 62.5mmol), replaced oxygen more than three times , 60 mL of anhydrous 1,4-dioxane was added, the temperature was raised to 110° C. and the reaction was performed for 30 hours. After the reaction was completed and cooled to room temperature, potassium carbonate (8.625 g, 62.5 mmol), methyl iodide (3.96 mL, 62.5 mmol) were added, stirred at room temperature for 4 hours, filtered under reduced pressure, the solvent and low boilers were evaporated in vacuo, and the crude product was Purified by column chromatography, the eluent is petroleum ether:ethyl acetate=10:1. The target intermediate D1 was obtained in the form of 1.74 g of a white so...
Embodiment 3
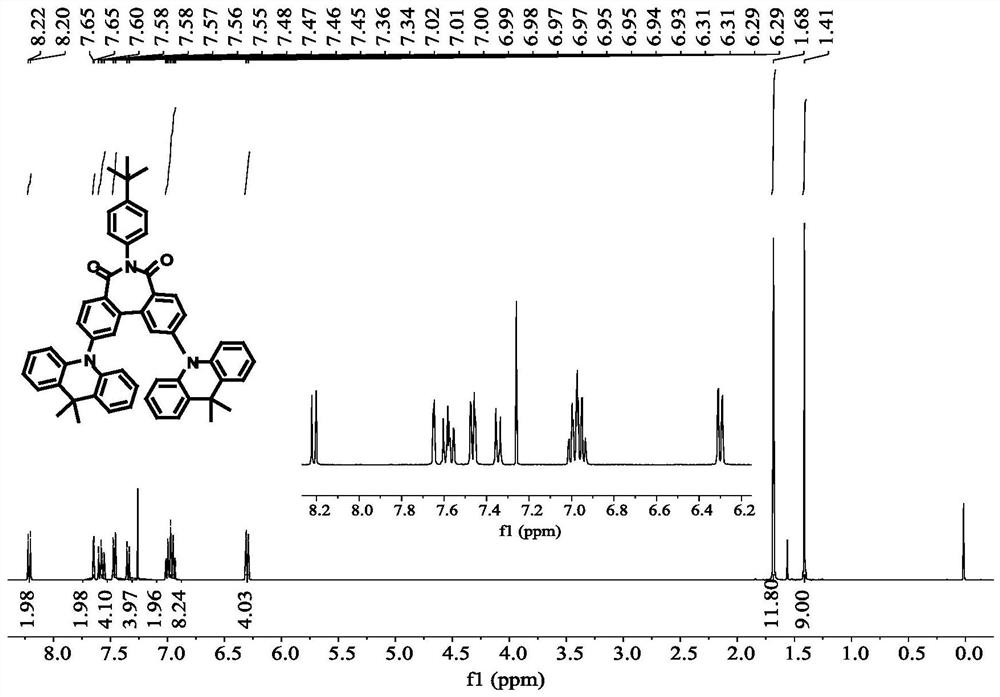
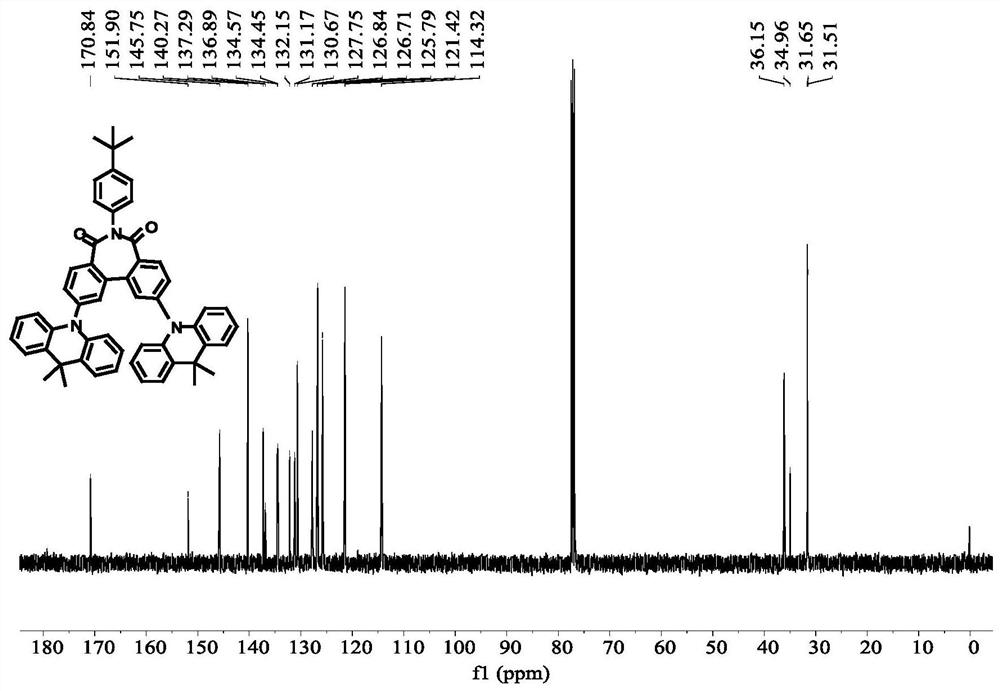
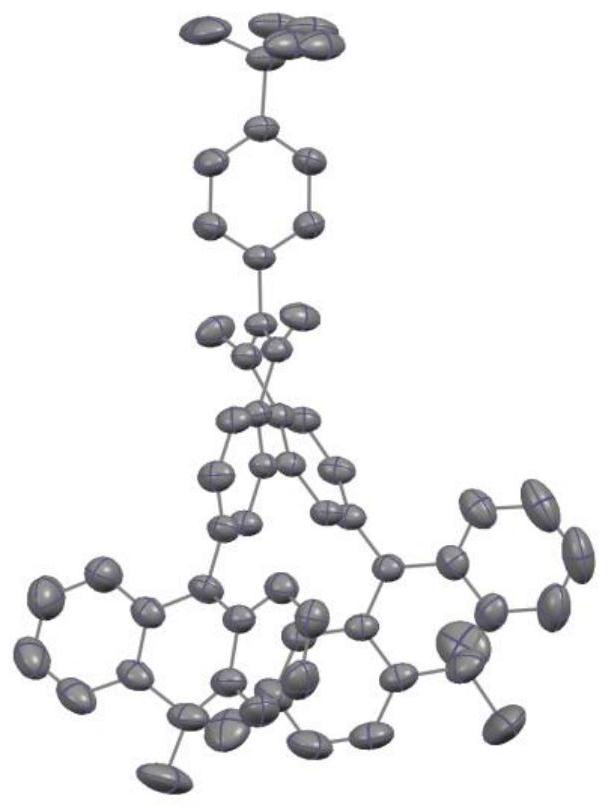
[0071] The preparation of compound C4 was similar to that of compound C1, except that 4-(9,9-dimethyl-9,10dihydroacridine)phenylboronic acid was used instead of 9,10-dihydro-9,9-di Methyl acridine was prepared to obtain intermediate E2, and then compound C4 was prepared as a pale yellow solid with a total yield of 37%.
[0072] High-resolution mass spectrometry, ESI source, positive ion mode, molecular formula [C 66 H 55 N 3 O 2 +Na] + , the theoretical value is 944.4186, and the measured value is 944.4182.
[0073] Preparation of Intermediate E2
[0074]
[0075] A magnetic stirring bar was placed in a 25mL high-pressure tube, Intermediate B1 (214mg, 0.5mmol), 4-(9,9-dimethyl-9,10dihydroacridine)phenylboronic acid (362mg, 1.1mmol) were added, Tetrakis(triphenylphosphine)palladium (57.8 mg, 0.05 mmol), potassium carbonate (276 mg, 2.0 mmol), nitrogen was replaced for more than three times, 5.0 mL of degassed tetrahydrofuran was added, and the reaction was refluxed for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com