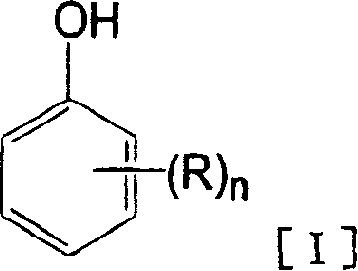
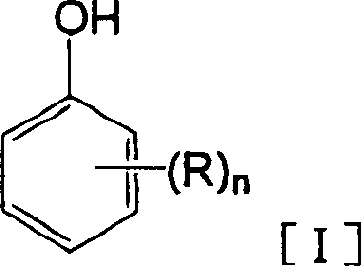
Process for production of hydroxybenzoic acids
A formic acid compound and a technology for producing hydroxybenzene, applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve the problems of expensive and high-cost aprotic polar organic solvents, and achieve high yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Feed 1155 g (5.6 mol) of 2,6-di-tert-butylphenol and 154 g (0.8 mol) of 28% sodium methoxide in methanol into a 2 L, stainless steel vessel equipped with a magnetic stirrer, thermometer, pressure gauge and alcohol separator middle. The resulting reaction mixture was heated to 180°C under nitrogen flow. At this temperature, the reaction was allowed to proceed for 5 hrs while distilling off the by-product, methanol. The resulting slurry of the sodium salt of 2,6-di-tert-butylphenol was heated to 210°C, and the nitrogen in the vessel was replaced with carbon dioxide. At a carbon dioxide pressure of 6kgf / cm 2 Carboxylation under (G) while stirring for 2 hr. After the reaction was completed, the reaction mixture was cooled to 90° C., and 1200 g of water was added thereto. The resulting mixture was heated to 85°C, and the mixture was separated into an aqueous layer and a solvent layer.
[0044] To the thus obtained aqueous layer was added a 73% sulfuric acid aqueous solu...
Embodiment 2
[0046] To the solvent layer separated from the aqueous layer in Example 1, 138 g (0.67 mol) of 2,6-di-tert-butylphenol and 154 g (0.8 mol) of 28% sodium methoxide in methanol were added. In the same manner as in Example 1, 173 g of 3,5-di-tert-butyl-4-hydroxybenzoic acid was obtained. The yield was 87% based on the amount of sodium methoxide fed.
[0047] This demonstrates that excess phenolic compounds can be reused as starting material for the process of the invention.
Embodiment 3
[0049] Using the same method as in Example 1, except that the reaction of 2,6-di-tert-butylphenol with sodium methoxide was carried out at 120° C., 164 g of 3,5-di-tert-butyl-4-hydroxybenzoic acid was obtained. The yield was 82% based on the amount of sodium methoxide fed.
[0050] This demonstrates that the hydroxybenzoic acid compound was obtained in high yield even if the reaction was carried out at a lower temperature.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com