Method of preparing heliotropin by using p-methyl phenol as raw material
A technology of p-cresol and jasmonal, which is applied in the direction of organic chemistry, can solve the problems of low total yield, low reaction yield, and easy agglomeration, and achieve low cost, ecological environment protection, and low price. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
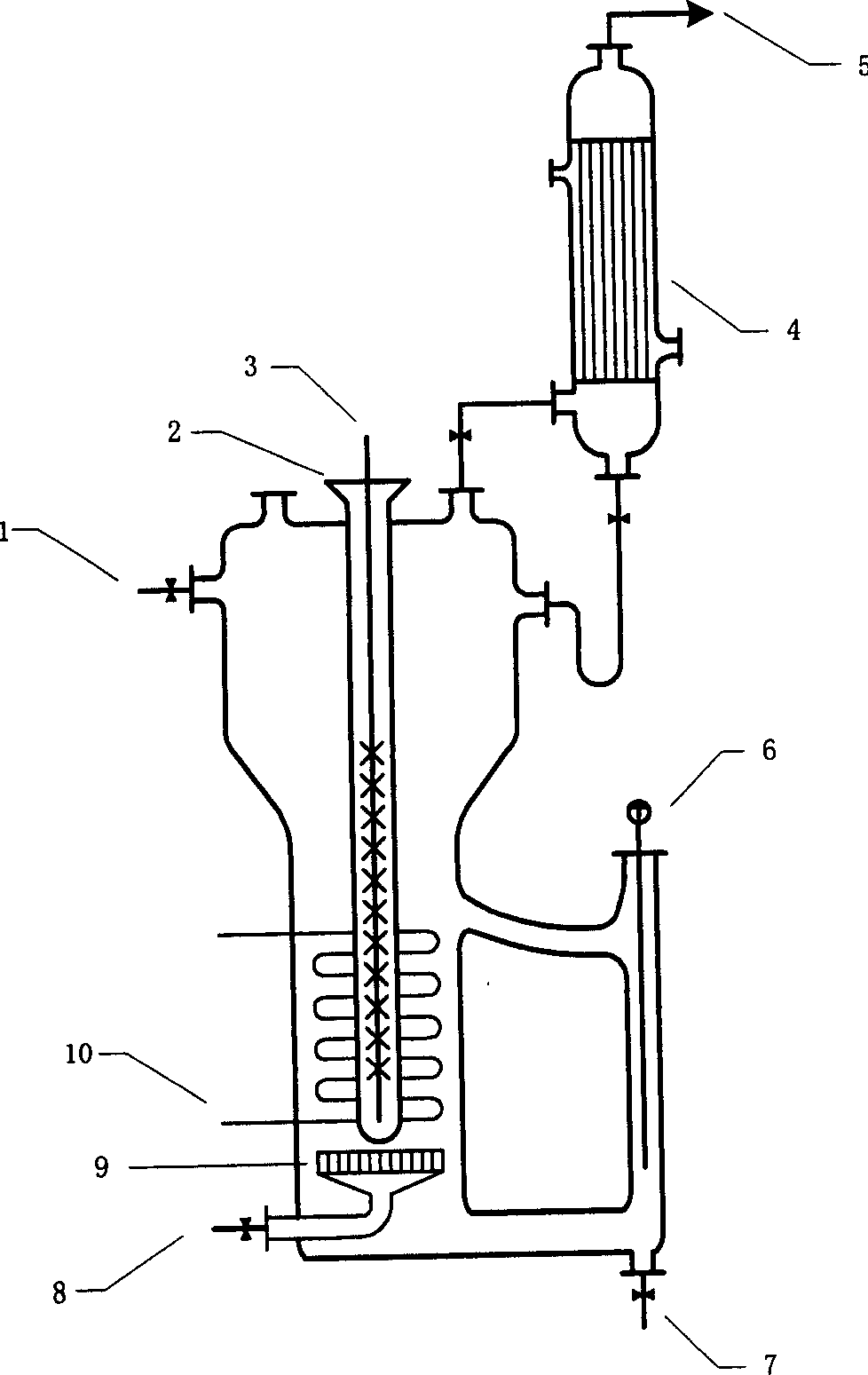
Image
Examples
Embodiment 1
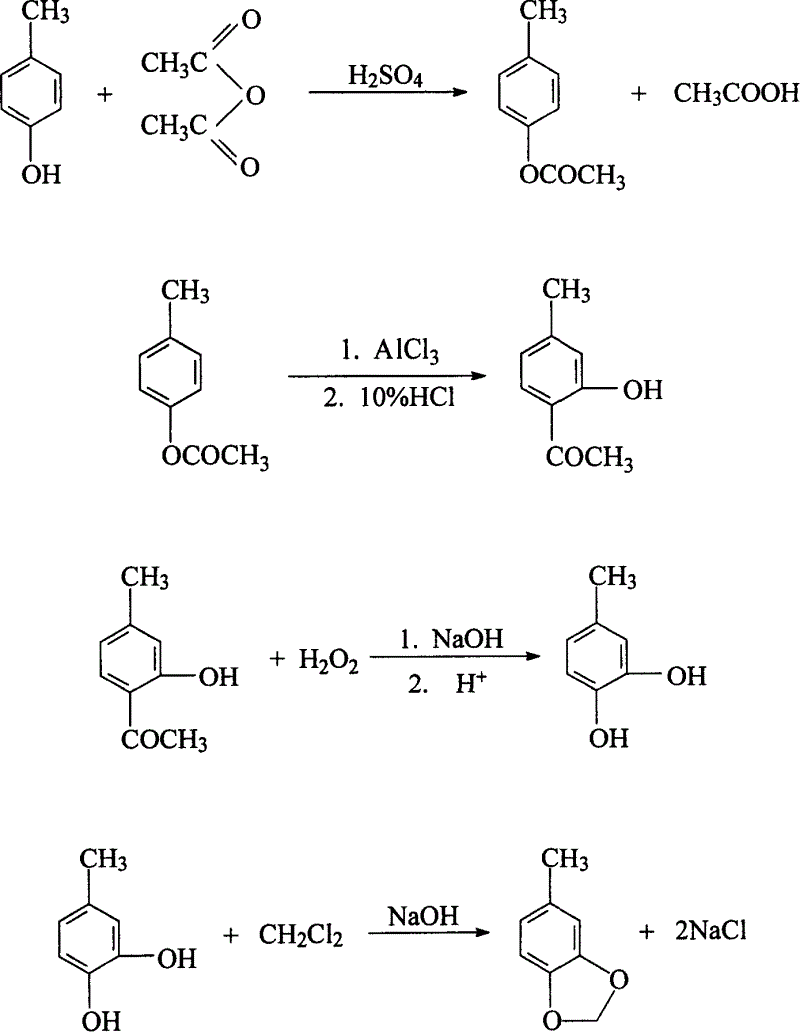
[0037] P-cresol 108g (1mole), acetic anhydride 408g (4mole), 98% H 2 SO 4 0.5 g was added into a four-necked reaction flask equipped with a stirrer, a thermometer, and a reflux condenser, and the temperature was raised to react under reflux for 1 hour. Distillation gave 138 g (0.92 mole) of the target product with a yield of 92%.
[0038] The product 138g (0.92mole) that last step obtains and AlCl 3160g (1.2 mole) was mixed, added to a four-necked reaction flask equipped with a stirrer, thermometer, feeder, and reflux condenser, heated to 90°C for 2 hours, and then heated to 160°C for 2 hours. After the reaction was completed, the mixed solution was cooled to 90° C., and the pH value of the system was adjusted to 5.2 with acid. Add 200 ml of toluene to extract the reaction solution twice into the system, combine the toluene phases, and recover the toluene to obtain 126.4 g (0.837 mole) of the target product, with a yield of 91%.
[0039] The product 126.4g (0.837mole) that...
Embodiment 2
[0045] P-cresol 216g (2.0mole), acetic anhydride 816g (8mole), 98% H 2 SO 4 1 g was added into a four-necked reaction flask equipped with a stirrer, a thermometer, and a reflux condenser, and the temperature was raised to react under reflux for 1.5 hours. Unreacted acetic anhydride and acetic acid produced by the reaction may be by-products. Distillation gave 279.6 g (1.864 mole) of the target product with a yield of 93%.
[0046] The product 279.6g (1.864mole) that last step obtains and AlCl 3 320g (2.4 mole) were mixed, added to a four-necked reaction flask equipped with a stirrer, thermometer, feeder, and reflux condenser, heated to 100°C for 2 hours, and then heated to 165°C for 3 hours. After the reaction was completed, the mixed solution was cooled to 100°C, and the pH value of the system was adjusted to 5.6°C by adding dilute acid. In the system, 400ml of toluene was added twice to extract the reaction solution, the toluene phase was combined, and the toluene was re...
Embodiment 3
[0053] 486g (4.5mole) of p-cresol, 1836g (18mole) of acetic anhydride, 98% H 2 SO 4 2 g was added into a four-necked reaction flask equipped with a stirrer, a thermometer, and a reflux condenser, and the temperature was raised to react under reflux for 2 hours. Distillation gave 619.5 g (4.13 mole) of the target product, with a yield of 91.7%.
[0054] The product 619.5g (4.13mole) that last step obtains and AlCl 3 720g (5.4 mole) were mixed, added to a four-necked reaction flask equipped with a stirrer, thermometer, feeder, and reflux condenser, heated to 110°C for 4 hours, and then heated to 170°C for 4 hours. After the reaction, the mixture was cooled to 100°C, and the pH of the system was adjusted to 5.8 with acid. 1000ml of toluene was added to the system twice to extract the reaction solution, the toluene phase was combined, and the toluene was recovered to obtain 569.4g (3.771 mole) of the target product, with a yield of 91.3%.
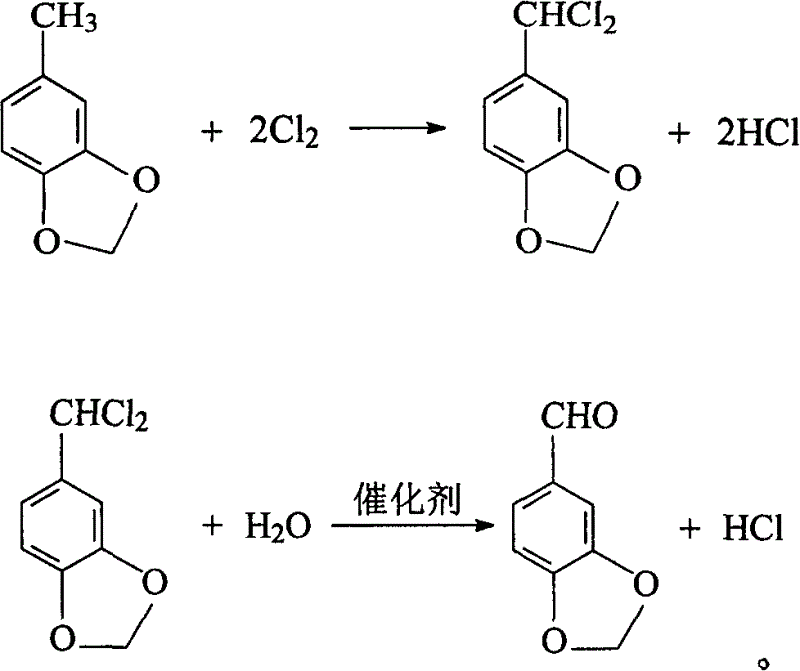
[0055] The product 569.4g (3.771mo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| power | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com