Separation and purification of high purity hemoglobin and virus inactivation technology
A hemoglobin, separation and purification technology, applied in the fields of hemoglobin/myoglobin, organic chemistry, peptide preparation, etc., can solve the key technology of adding excessive non-toxic antioxidants, unable to achieve virus inactivation, low hemoglobin purity, etc. problems, to achieve the effect of shortening the time period, reducing production costs, and simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
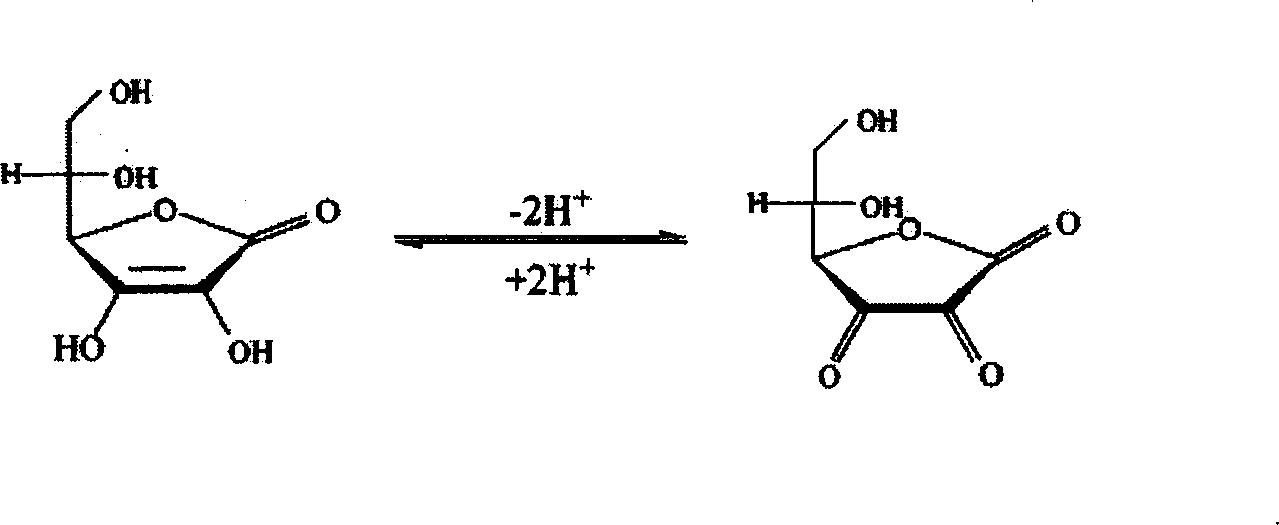
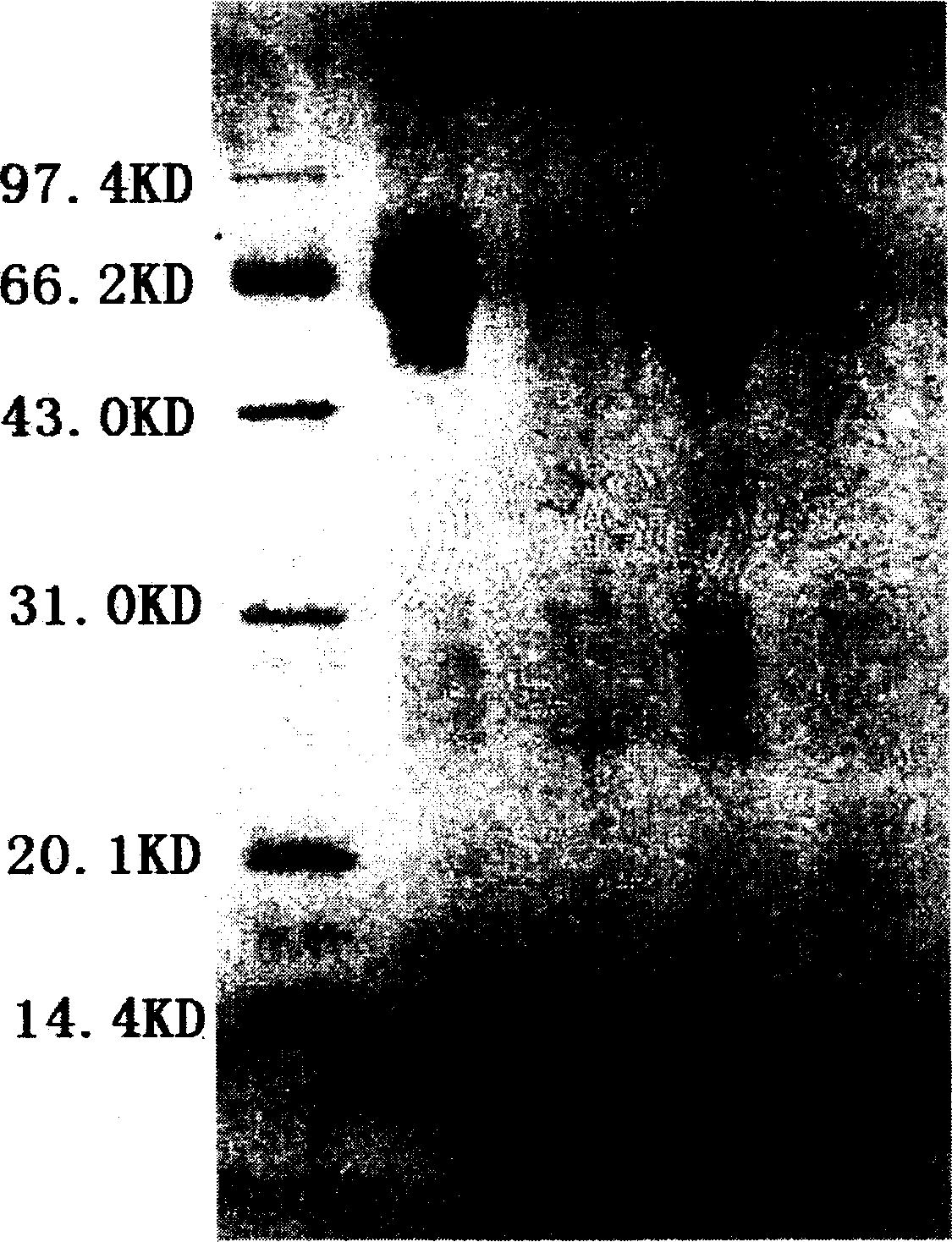
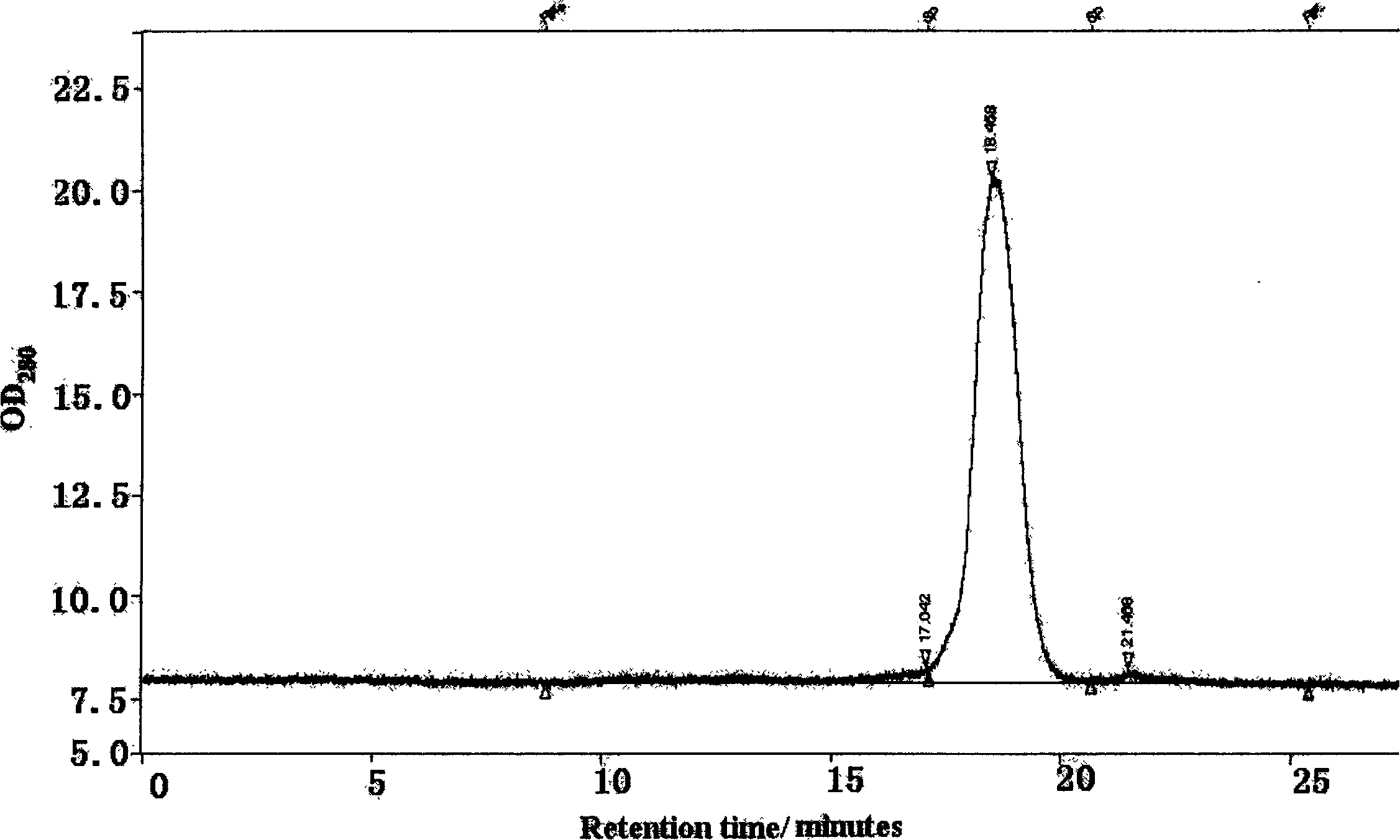
[0033]Embodiment: A separation and purification and virus inactivation process of high-purity hemoglobin is characterized in that it is composed of the following steps: the placental blood collected is subjected to a pyrogen-free physiological method at a low temperature of 4°C according to a conventional method. After repeated washing with saline and centrifugation, packed erythrocytes were obtained, and then hemolyzed with phosphate buffer solution with an osmotic pressure of 15 mOsmol and a pH of 7.4 to obtain crude hemolyzed blood. Add conventional bacteriostatic drugs such as penicillin and streptomycin in the amount of 100,000 units / 1000 ml to the crude hemolysate and mix well, then add 3% vitamin C solution to adjust the pH of the hemolysate to 6.00. The mixed solution was first pumped with a vacuum pump, and the vacuum degree on the surface of the solution was reduced to 0, and kept in this state for 15 minutes; then, high-purity nitrogen gas without oxygen was introduc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com