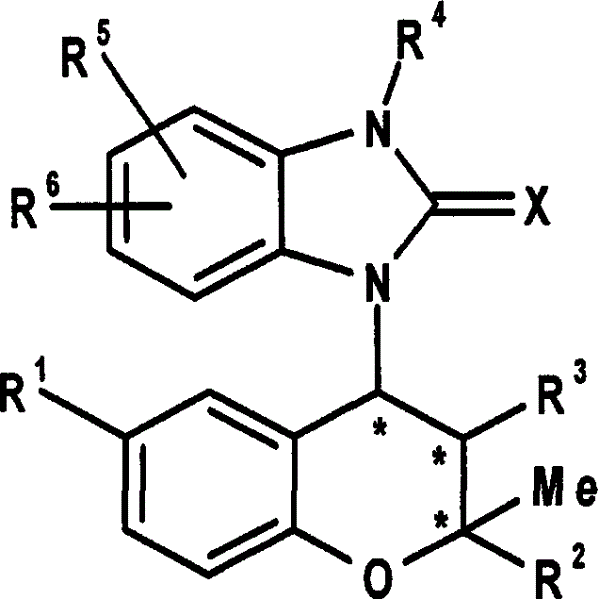
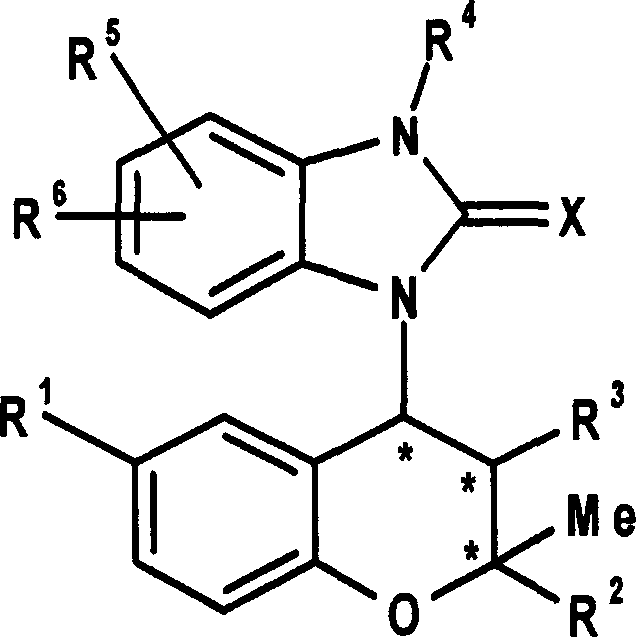
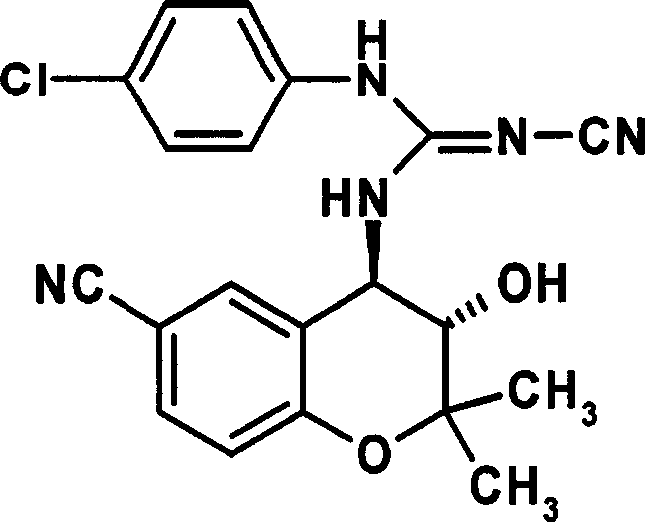
Benzopyran derivatives substituted with a benzimidazole derivative, pharmaceutically acceptable salts thereof, their preparations and pharmaceutical compositions containing them
A technology of benzopyran derivatives and benzimidazoles, which can be used in drug combination, organic chemistry, metabolic diseases, etc., and can solve problems such as difficult development of new drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0125] Example 1: (2S, 3R, 4S)-6-nitro-3,4-dihydro-3-hydroxyl-2-dimethoxymethyl-2-methyl-4-(2,3-di Preparation of Hydrogen-2-cyanoimino-1H-benzimidazol-1-yl)-2H-1-benzopyran
[0126] (2S, 3R, 4S)-6-nitro-3,4-dihydro-3-hydroxy-2-dimethoxymethyl-2-methyl-4-[(2-aminobenzene Preparation of base)amino]-2H-1-benzopyran
[0127] 950 mg (3.38 mmol) of epoxy compound (2S, 3R, 4R)-6-nitro-3,4-dihydro-3,4-epoxy-2-dimethoxymethyl-2-methyl- 2H-1-benzopyran and 370mg (3.38mmol) of 1,2-phenylenediamine were dissolved in 3ml of acetonitrile (CH 3 CN), and then add 754mg (3.38mmol) magnesium chlorate [Mg(ClO 4 ) 2 ]. The reaction was stirred at room temperature for 2 h, and 10 ml of saturated NaHCO was added 3 solution, and the aqueous layer was extracted with 30 ml of ethyl acetate. The combined organic layers were washed with anhydrous MgSO 4 Dry, filter and concentrate under reduced pressure. The residue was purified by column chromatography (hexane:ethyl acetate=1:1) to obtain 67...
Embodiment 2
[0132] Example 2: (2R, 3R, 4S)-6-nitro-3,4-dihydro-3-hydroxyl-2-dimethoxymethyl-2-methyl-4-(2,3-di Preparation of Hydrogen-2-cyanoimino-1H-benzimidazol-1-yl)-2H-1-benzopyran
[0133] (2R, 3R, 4S)-6-nitro-3,4-dihydro-3-hydroxy-2-dimethoxymethyl-2-methyl-4-[(2-aminobenzene Preparation of base)amino]-2H-1-benzopyran
[0134] Using a method similar to Example 1 step 1, 540mg (1.92mmol) epoxy compound (2R, 3R, 4R)-6-nitro-3,4-dihydro-3,4-epoxy-2-dimethyl Oxymethyl-2-methyl-2H-1-benzopyran and 208 mg (1.92 mmol) of 1,2-phenylenediamine were reacted to obtain 404 mg of the target compound (yield: 54%).
[0135] 1 H NMR (200MHz, CDCl 3 )δ1.43(s, 3H), 3.50(s, 3H), 3.55(s, 3H), 4.14(d, 1H), 4.45(s, 1H), 4.49(d, 1H), 6.75(m, 5H ), 8.09(dd, 1H), 8.32(d, 1H)
[0136] (2R, 3R, 4S)-6-nitro-3,4-dihydro-3-hydroxy-2-dimethoxymethyl-2-methyl-4-(2,3-di Preparation of Hydrogen-2-cyanoimino-1H-benzimidazol-1-yl)-2H-1-benzopyran
[0137] 404 mg (1.04 mmol) of the compound prepared in the a...
Embodiment 3
[0139] Example 3: (2S, 3S, 4R)-6-nitro-3,4-dihydro-3-hydroxyl-2-dimethoxymethyl-2-methyl-4-(2,3-di Preparation of Hydrogen-2-cyanoimino-1H-benzimidazol-1-yl)-2H-1-benzopyran
[0140] (2S, 3S, 4R)-6-nitro-3,4-dihydro-3-hydroxy-2-dimethoxymethyl-2-methyl-4-[(2-aminobenzene Preparation of base)amino]-2H-1-benzopyran
[0141] 2g (7.11mmol) epoxy compound (2S, 3S, 4S)-6-nitro-3,4-dihydro-3,4-epoxy-2-dimethoxymethyl-2-methyl- 2H-1-benzopyran and 1.15 g (10.7 mmol) of 1,2-phenylenediamine were reacted in a manner similar to that described in Step 1 of Example 1 to obtain 2.08 g of the target compound (yield: 75%) .
[0142] 1 H NMR (200MHz, CDCl 3 )δ1.36(s, 3H), 3.58(s, 3H), 3.59(s, 3H), 4.23(d, 1H), 4.41(s, 1H), 4.51(d, 1H), 6.72-6.78(m , 4H), 6.90(d, 1H), 8.03(dd, 1H), 8.34(d, 1H)
[0143] Mass spectrum: 389, 296, 119, 108, 75
[0144] (2S, 3S, 4R)-6-nitro-3,4-dihydro-3-hydroxy-2-dimethoxymethyl-2-methyl-4-(2,3-di Preparation of Hydrogen-2-cyanoimino-1H-benzimidazol-1-yl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com