2,3,4,5-tetramethoxyl toluene synthesis method
A technology of tetramethoxytoluene and synthesis method, which is applied in the field of chemical synthesis technology, and can solve problems such as unsuitable for industrial production, difficult purification of products, and inconvenient operation.
Inactive Publication Date: 2011-01-05
JIANGSU YABANG PHARMA RES & DEV
View PDF0 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The synthetic preparation route is inconvenient in operation, the yield is not high, and the product is not easy to purify, so it is not suitable for industrial production
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
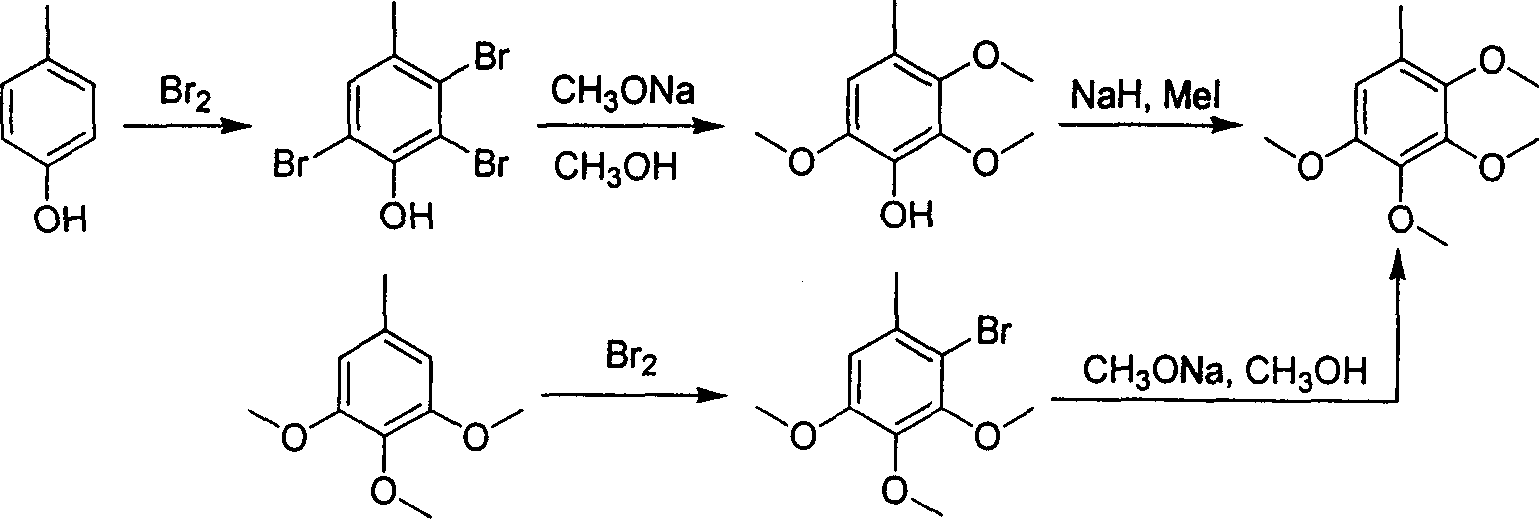
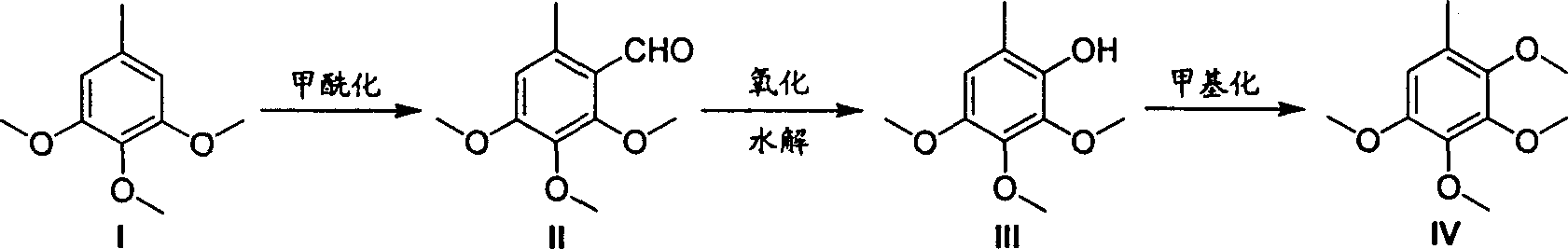
The invention relates to a method for synthesizing 2, 3, 4, 5-four metonym toluene, which comprises the following steps: using 3, 4, 5-three metonym toluene as starting raw material, obtaining the intermediate 2, 3, 4-three metonym-6-methxyl benzaldehyde after 2-bit formylated, obtaining 2, 3, 4-three metonym-6-methxyl benzene after oxidizing and obtaining 2, 3, 4, 5-four metonym toluene after methylamine. The methylamine reagent is cheesed form methyl iodide, methyl sulfate, methyl carbonate, and azimethane; the base is cheesed form caustic soda, caustic potash, potash and sodium hydride; the reagent is cheesed form alcohol, tetrahydropyran, acetone and propylidene chloride; the oxidant is cheesed form hydrogen diode solution and perbenzoic acid and so on. The formylation is N, N-dimethyl formamide solution with the drop phosphorus trichloride temperature below 50 deg. and the reacting temperature 60 deg.-100 deg.
Description
The synthetic method of 2,3,4,5-tetramethoxytoluene technical field The invention relates to a chemical synthesis process, in particular to a synthesis method of a pharmaceutical chemical intermediate 2,3,4,5-tetramethoxytoluene. Background technique 2,3,4,5-Tetramethoxytoluene is a very important pharmaceutical and chemical intermediate, mainly used as a raw material for the synthesis of coenzyme Q series products (Q0-10). The synthesis method of 2,3,4,5-tetramethoxytoluene reported in the literature includes: taking p-cresol as raw material, after bromine bromination, methoxylation and methylation to obtain 2,3,4, 5-tetramethoxytoluene, or 3,4,5-trimethoxytoluene as raw material, after bromine bromination, methoxylation to obtain 2,3,4,5-tetramethoxytoluene. The synthetic route is shown in the figure below (J.Org.Chem.1987, 52, 3872-3875). The synthetic preparation route is inconvenient to operate, the yield is not high, the product is not easy to purify, and is not su...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07C43/235C07C41/01
Inventor 陈再新蒋龙刘襄张英
Owner JIANGSU YABANG PHARMA RES & DEV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com