Method for preparing carbon nanotube-protein stationary phase
A technology for immobilizing carbon nanotubes and proteins, which is applied in the nanometer field, can solve problems not involved in the application of microfluidic chips, and achieve the effects of increasing the amount of immobilization, good stability, and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment one. the preparation method I of protein stationary phase
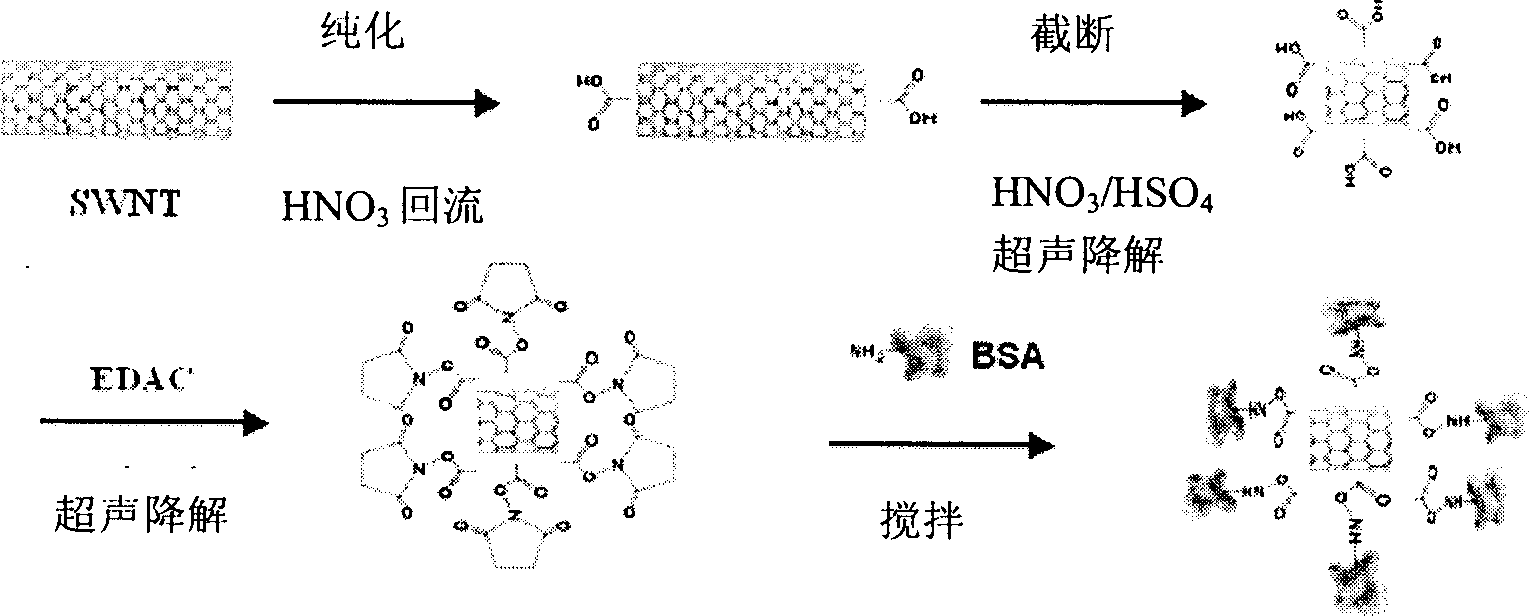
[0030] (1) Purified carbon nanotubes
[0031] Put 1g of SWNTs in a round bottom flask, add 16ml concentrated HNO 3 , reflux in an oil bath at 140°C for 4 hours, dilute with water, centrifuge, discard the supernatant, repeat washing and centrifugation until the supernatant pH ~ 6, filter with a microporous membrane with a pore size of 0.22 μm to obtain a black solid, dry for later use .
[0032] (2) Truncated nanotubes
[0033] Take 50 mg of the above-mentioned purified SWNTs in 45 ml of concentrated HNO 3 and concentrated H 2 SO 4 Sonicate in (1:3v / v) for 8 hours, dilute, filter, wash the solid until the filtrate is close to neutral, and dry the solid for later use.
[0034] (3) Activation of nanotubes and covalent binding of proteins
[0035] Weigh 0.075 mg of the cut-off SWNTs above, add 1 ml PBS, sonicate for 5 minutes, add 0.4 mg EDAC and sonicate for 2 hours, continue to add 0.9 mg BSA, a...
Embodiment 2
[0036] Embodiment two. The preparation method II of protein stationary phase
[0037] The difference from the preparation method I of the protein stationary phase is that the above cut-off 0.075mg SWNTs was added to 1ml PBS, sonicated for 5 minutes, 0.3mg EDC and 0.75mg NHS were added and sonicated for 2 hours, and 0.9mg BSA was added, and stirred for 24 hours Then the protein stationary phase is obtained.
Embodiment 3
[0038] Example three. Separation of tryptophan enantiomers.
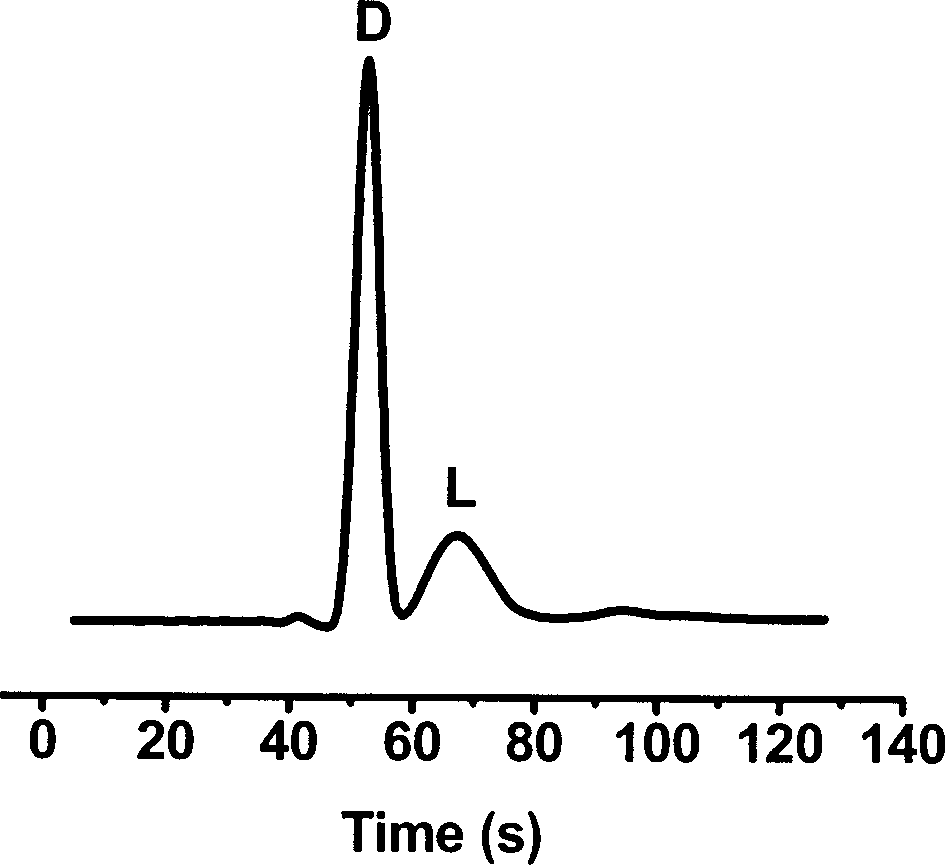
[0039] Using the BSA stationary phase prepared in the above example, use a micro-sampler to fill the PMMA chip modified with polymers, place it in a refrigerator at 4°C to dry, use phosphate buffer as the mobile phase, and tryptophan enantiomers can be separated . The separation conditions are as follows: the separation channel length of the chip is 3.2 cm; the separation field strength is +218 V / cm; 20 mM phosphate buffer solution, pH=7.4; 24 μM tryptophan; and the voltage is 0.6 V for constant potential detection. figure 2Separation of tryptophan enantiomers is shown. It can be seen that tryptophan isomers can be baseline-separated within 70 seconds in the chip channel with BSA protein stationary phase, and the resolution is about 1.28. This result shows that the protein stationary phase prepared according to the invention can not only be stably fixed in the channel of the chip, but also preserve the original c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com