Production process of human-alpha phylaxin-1 protein with colibacillus
A technology of Escherichia coli and defensin, which is applied in the field of producing human alpha defensin 1 protein by using Escherichia coli, can solve problems such as unreported, achieve the effects of good transcription and translation efficiency, short development period, and easy processing and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Chemical Synthesis and Cloning of α-Defensin 1 Mature Peptide Coding Sequence
[0052] 1. Optimal design and chemical synthesis of HNP1 mature peptide coding sequence
[0053] The HNP1 mRNA sequence was obtained from Genbank ( NM_004084 ) and its mature peptide amino acid sequence ( P59665 ), thus deducing its mature peptide coding sequence (90bp). On the basis of sequence sequence analysis, the original sequence is optimized and designed according to the differences between biologically preferred codons and their rare codons. That is, the rare codons in HNP1 were deleted and replaced with codons favored by E. coli. In order to achieve a good translation termination effect, two stop codons, TGA and TAA, were added at the end of the optimized coding sequence to facilitate the expression of the target protein in E. coli.
[0054] The modified sequence is shown in Table 2.
[0055] The two complementary strands of the redesigned HNP1 mature peptide coding ...
Embodiment 2
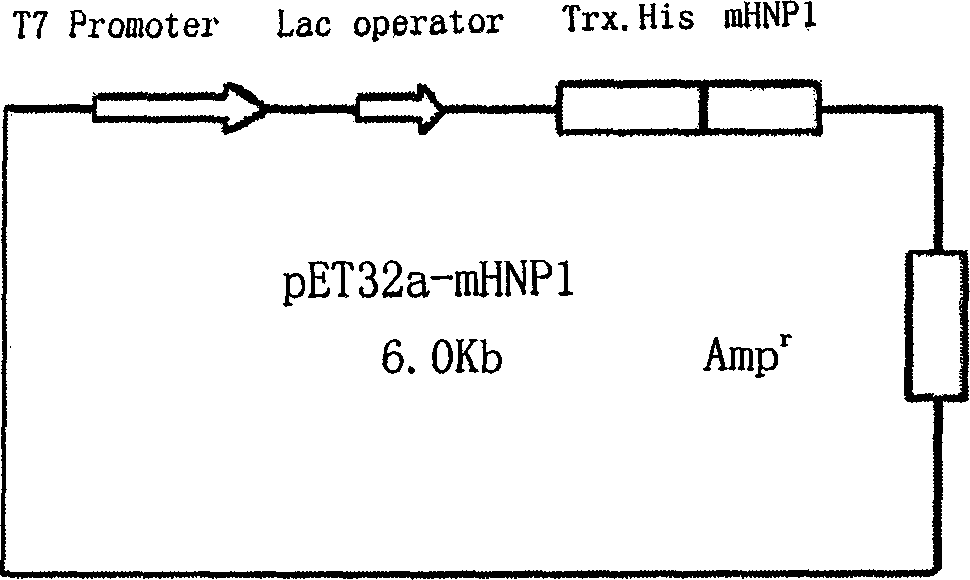
[0087] Example 2: Construction, induced expression and activity identification of mHNP1 T7 expression vector
[0088] 1. Construction of mHNP1 T7 expression vector
[0089] (1) Strains and plasmids:
[0090] Escherichia coli BL21(DE3)pLysS was preserved in our laboratory. pET32a plasmid (Novagen, see Figure 4 ) was a gift from Dr. Zeng Xiankun, School of Medicine, Southeast University.
[0091] (2) Tool enzymes and reagents:
[0092] Restriction enzyme, T 4 Ligase and Taq DNA polymerase were purchased from Dalian Bao Biological Engineering Co., Ltd., Shanghai Sangong Co., Ltd., and Beijing Dingguo Biotechnology Co., Ltd., respectively.
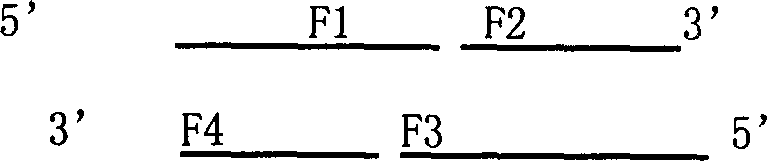
[0093] (3) Design and synthesis of primers:
[0094] The PCR primers were optimized and designed according to the present invention to design the coding sequence of mHNP1 mature peptide, all of which were synthesized by Dalian Bao Biology Co., Ltd. The primer sequences are as follows:
[0095] P1 5'GCG GGA TCC ATG GCC TGC TAT TGC C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com