A method for splitting racemic leucine
A technology for spinning leucine and leucine, which is applied in the field of splitting racemic leucine, can solve the problems of being unsuitable for industrial production, increasing production costs, prolonging the production cycle, etc., and achieves convenient treatment and purification, easy hydrolysis, The effect of high equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
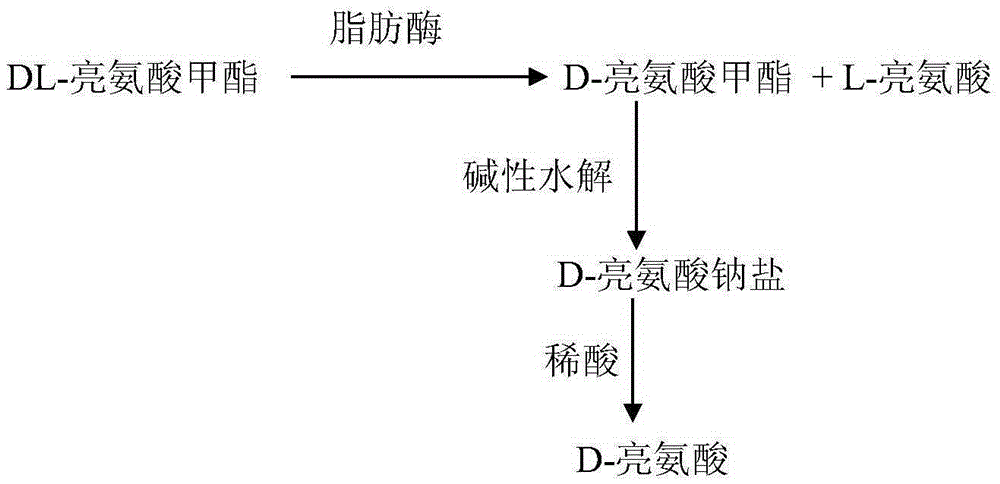
[0024] Weigh 3.62g (0.02mol) of leucine methyl ester hydrochloride into the small conical flask, then weigh 1.68g (0.02mol) of sodium bicarbonate into the conical flask, and measure 16ml of saturated lipase solution into the In the reaction flask, put the Erlenmeyer flask into a constant temperature oscillator to vibrate, set the reaction temperature to 38° C., react for 8 hours, then slowly cool down the reaction flask, and vacuum filter. Obtaining filter cake is L-leucine 1.02g, productive rate is 77.86%, specific rotation [α] D 20 =+14.6°(C=2, 6mol.L -1 HCl). The filtrate contains D-leucine methyl ester, add a certain amount of sodium hydroxide to the filtrate, add an equal volume of methanol after dissolving, stir at room temperature for 10h, and use 1mol.L -1 Adjust the pH of the solution to 6 with dilute hydrochloric acid, react for another 1 h, filter, wash the filter cake with water, and dry to obtain 0.90 g of D-leucine with a yield of 68.70%, [α] D 20 =-14.35°(C...
example 2
[0026] Take by weighing 3.62g leucine methyl ester hydrochloride and add in the small conical flask, then take by weighing 1.68g (0.02mol) sodium bicarbonate and add in the conical flask, and measure 16ml saturated lipase solution and add in the reaction flask, The Erlenmeyer flask was placed in a constant temperature oscillator to vibrate, the reaction temperature was 34° C., and the reaction time was 8 hours. Then, the reaction flask was cooled down slowly and vacuum filtered. Obtain filter cake and be L-leucine 0.92g, productive rate is 70.22%, specific rotation [α] D 20 =+14.6°(C=2, 6mol.L -1 HCl). The filtrate contains D-leucine methyl ester, add a certain amount of sodium hydroxide to the filtrate, add an equal volume of methanol after dissolving, stir at room temperature for 10h, and use 1mol.L -1 Adjust the pH of the solution to 6 with dilute hydrochloric acid, react for another 1 h, filter, wash the filter cake with water, and dry to obtain 0.94 g of D-leucine with...
example 3
[0028]Weigh 3.62g of leucine methyl ester hydrochloride and add it to the small conical flask, then weigh 1.68g of sodium bicarbonate and add it to the conical flask, and measure 16ml of saturated lipase solution and add it to the reaction flask. Put it into a constant temperature shaker to vibrate, set the reaction temperature to 38°C, react for 9 hours, then slowly cool down the reaction bottle, and vacuum filter. The obtained filter cake is L-leucine 1.19g, specific rotation [α] D 20 =+14.6°(C=2, 6mol.L -1 HCl). The filtrate contains D-leucine methyl ester, add solid sodium hydroxide to the filtrate, add methanol after dissolving, stir continuously for 10h, and use 1mol.L -1 Adjust the pH of the solution to 6 with dilute hydrochloric acid, react for another 1 h, filter, wash the filter cake with water, and dry to obtain 0.83 g of D-leucine with a yield of 63.36%, [α] D 20 =-14.40°(C=2, 6mol.L -1 HCl).
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com