Method for identifying finger print atlas of injecta
A fingerprint and comparison fingerprint technology is applied in the field of quality control of traditional Chinese medicine compositions, which can solve the problems of irregular and perfect quality standards of traditional Chinese medicines, inability to accurately reflect the quality status of traditional Chinese medicines, and backward quality analysis and evaluation methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
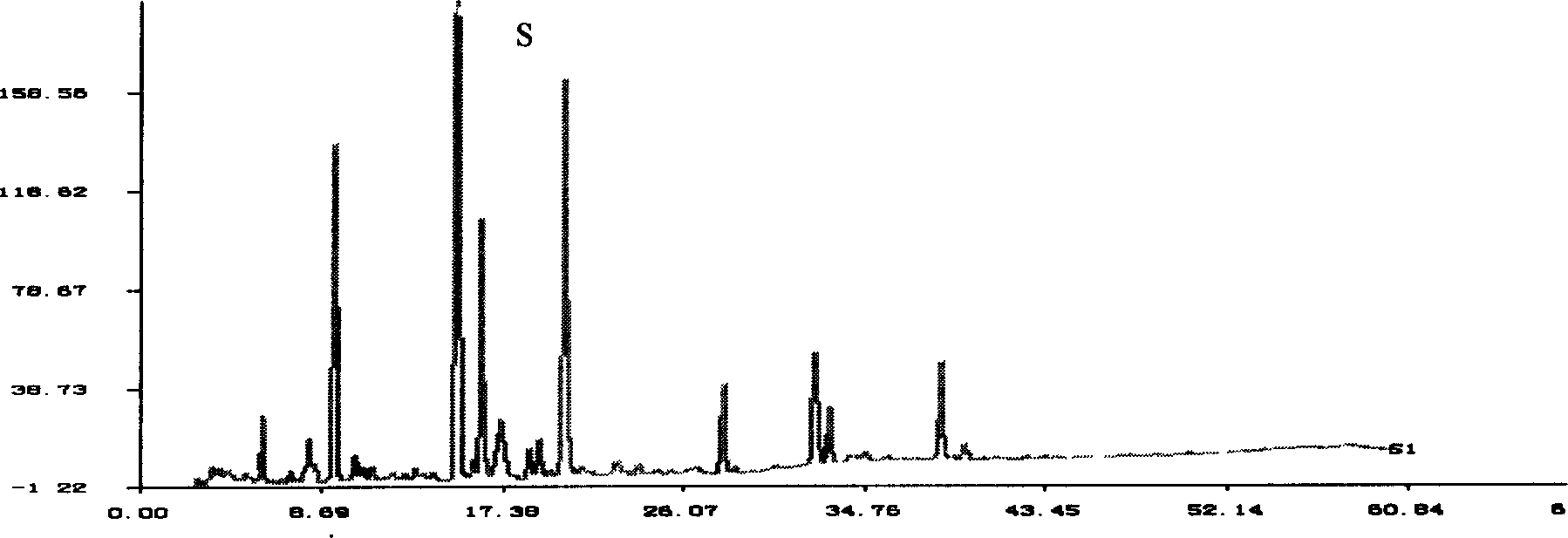
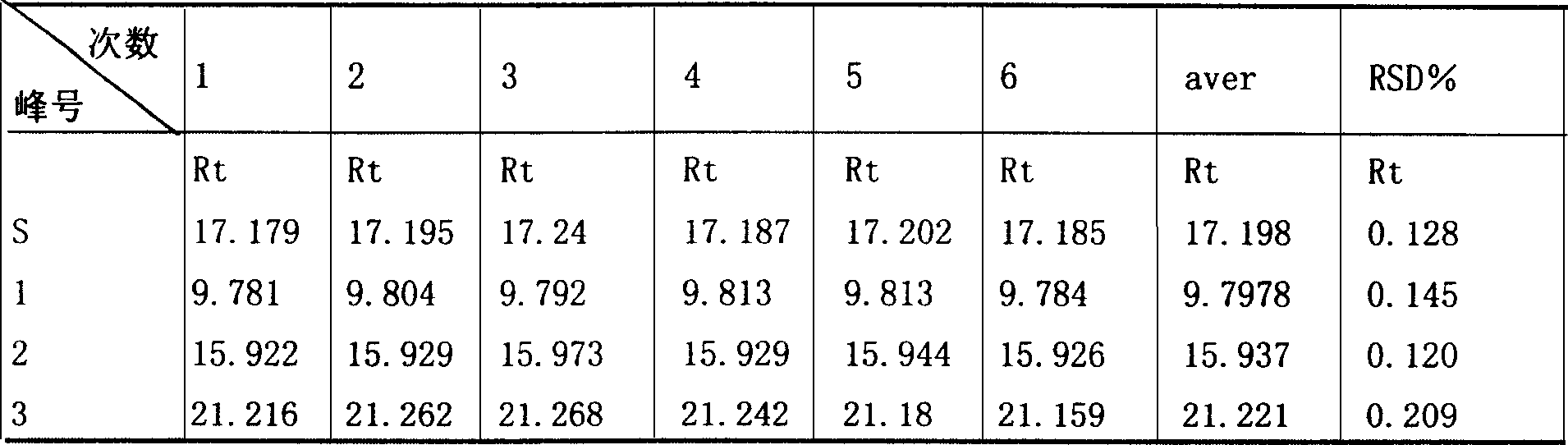
[0067] Embodiment 1: Reduning Injection Liquid Phase Fingerprint Determination Method
[0068] time (minutes)
Methanol (%)
0.1% phosphoric acid (%)
0
50
60
20
60
60
80
40
40
[0069] The flow rate is 0.8ml / min; the detection wavelength is 225nm. The number of theoretical plates is calculated according to the peak of the reference substance (geniposide), and should not be less than 6000.
[0070] Preparation of reference substance solution: Take an appropriate amount of geniposide reference substance, weigh it accurately, add methanol to make a solution containing 50 μg per 1 ml, and obtain it.
[0071] Preparation of the test solution: Take 1ml of this product, put it in a 25ml measuring bottle, add 50% methanol solution to dilute to the mark, shake well, and you get it.
[0072] Determination method: Precisely draw 10 μl of the reference substance solution and the test solution, inject them into the liquid...
Embodiment 2
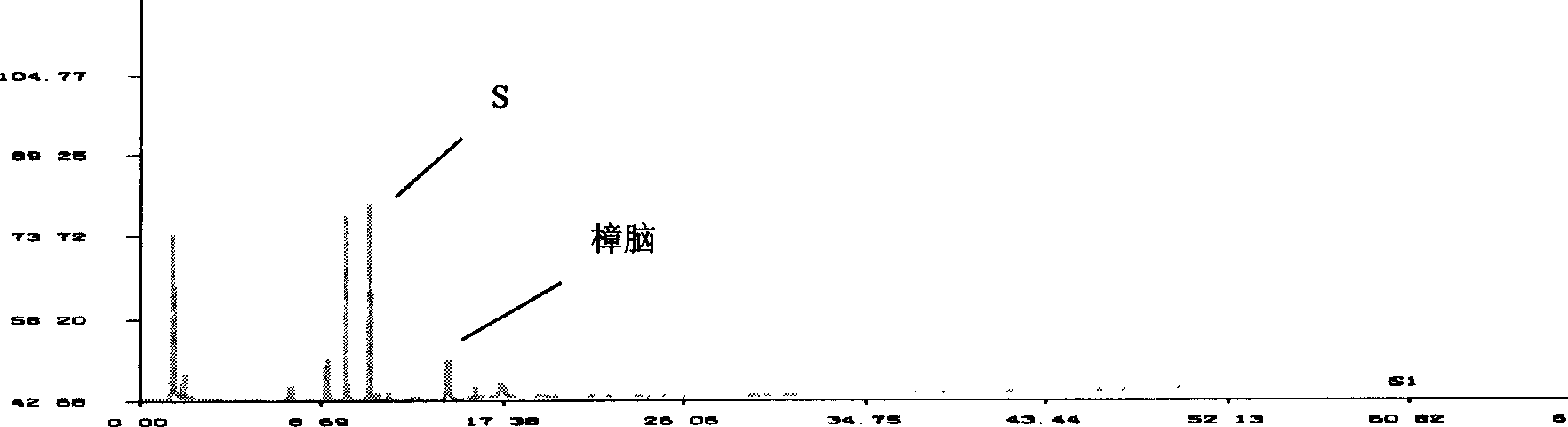
[0075]Embodiment 2: Determination method of reduning injection gas phase fingerprint
[0076] Chromatographic conditions and system adaptability test Agilent 6890 gas chromatograph, gas chromatographic headspace sampling method determination, headspace oven temperature 95 ° C, transfer tube temperature 110 ° C, sampling loop temperature 125 ° C, equilibration time: 60 min; with 5 % diphenyl-95% dimethylsilane copolymer as the stationary phase capillary chromatographic column (HP-5: 30m×0.32mm×0.25μm); inlet temperature: 240°C; detector temperature (FID): 240 ℃; headspace sampling measurement, split ratio: 1:1; temperature program: initial temperature 50 ℃, keep 5 minutes, rise to 80 ℃ at 10 ℃ per minute, keep 10 minutes, rise to 130 ℃ at 10 ℃ per minute , kept for 6 minutes, then raised to 240°C at 5°C per minute, held for 9 minutes, and measured; carrier gas flow rate: 2.0ml / min; the number of theoretical plates was calculated according to the peak of the reference substance ...
Embodiment 3
[0082] Embodiment 3: Reduning Injection Biphasic Fingerprint Determination Method
[0083] time (minutes)
Methanol (%)
0.1% phosphoric acid (%)
0
50
60
20
60
60
80
40
40
[0084] The flow rate is 0.8ml / min; the detection wavelength is 225nm. The number of theoretical plates is calculated according to the peak of the reference substance (geniposide), and should not be less than 6000.
[0085] Preparation of reference substance solution: Take an appropriate amount of geniposide reference substance, weigh it accurately, add methanol to make a solution containing 50 μg per 1 ml, and obtain it. Preparation of the test solution: Take 1ml of this product, put it in a 25ml measuring bottle, add 50% methanol solution to dilute to the mark, shake well, and you get it. Determination method: Precisely draw 10 μl of the reference substance solution and the test solution, inject them into the liquid chromatograph respect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com