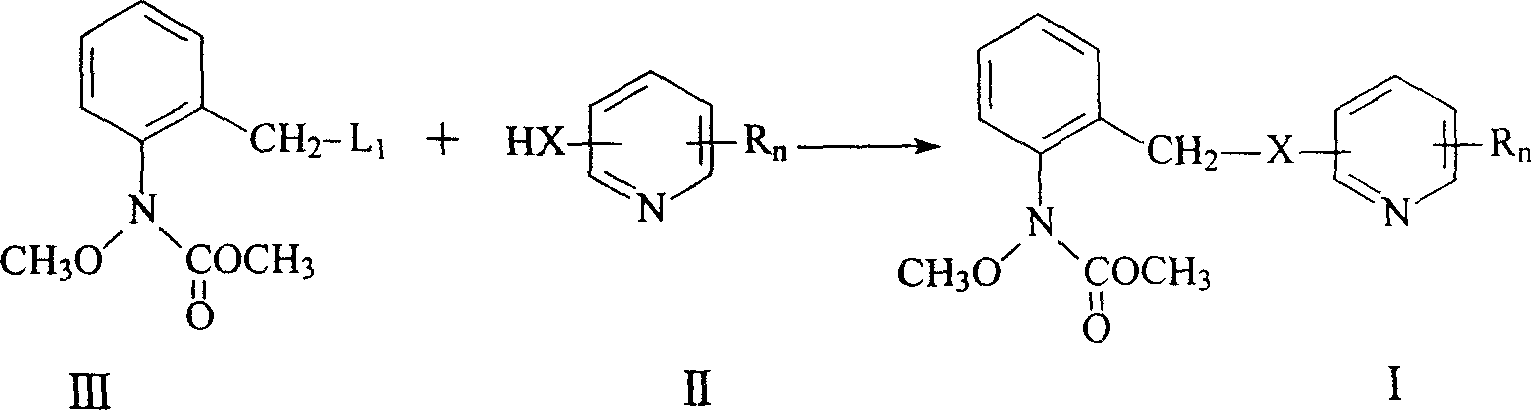
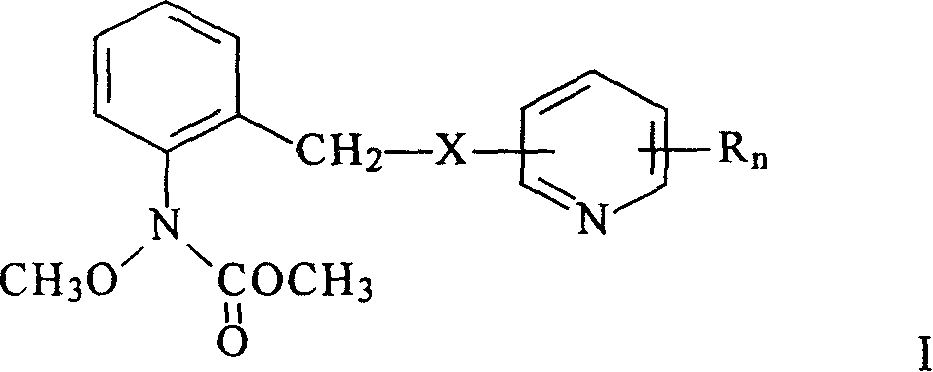N-(2-substituted phenyl)-N-methoxy carbamate compounds and their preparation and use
A kind of methoxycarbamic acid and compound technology, applied in N--N-methoxycarbamic acid methyl ester compound and its preparation and application field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: Preparation of methyl N-methoxy-N-[2-[[(3,5,6-trichloropyridin-2-yl)oxy]methyl]phenyl]carbamate (compound 13)
[0060] Reaction formula:
[0061]
[0062] With 0.4 gram (2mmol) 3,5,6-trichloropyridinol (commercially available) and 0.6 gram (2.2mmol) methyl N-2-bromomethylphenyl-N-methoxycarbamate (with reference to WO02 / 46142) was dissolved in 5 ml of dimethylformamide, 0.45 g of potassium carbonate was added, and the reaction was stirred at room temperature for 6 hours.
[0063] The reaction solution was absorbed in ethyl acetate, washed with water, washed with saturated brine and dried over anhydrous sodium sulfate, and then precipitated under reduced pressure. 0.55 g of a light yellow solid was obtained as compound 13 by separation on a silica gel column. Content: 95%, melting point: 94.0-94.7°C.
Embodiment 2
[0064] Example 2: Preparation of methyl N-methoxy-N-[2-[[(2,6-difluoro-3,5-dichloropyridin-4-yl)amino]methyl]phenyl]carbamate (compound 17)
[0065] Reaction formula:
[0066]
[0067] 0.43 g (2.5 mmol) of 4-amino-2,6-difluoro-3,5-dichloropyridine and 0.77 g (2.8 mmol) of N-2-bromomethylphenyl-N-methoxycarbamate The ester was dissolved in 5 ml of dimethylformamide, 1.0 g of potassium carbonate was added, and the reaction was stirred at 30-35°C for 4 hours.
[0068] The reaction solution was absorbed in ethyl acetate, washed with water, washed with saturated brine and dried over anhydrous sodium sulfate, and then precipitated under reduced pressure. 0.6 g of a light yellow solid was obtained as compound 17 by separation on a silica gel column. Content: 95%, melting point: 82-83°C.
[0069] According to the above method, other compounds in the general formula I can be obtained by appropriately changing the starting compound.
[0070] NMR data of some compounds ( 1 HNMR,...
Embodiment 3
[0075] Embodiment 3 60% wettable powder
[0076] Compound 13 60%
[0077] Sodium dodecylbenzenesulfonate 1%
[0078] Sodium lignosulfonate 6%
[0079] Diffuser NNO 5%
[0080] Diatomaceous earth make up to 100%
[0081] All components (all solid) are mixed together and pulverized in a pulverizer until the fineness reaches the standard (≤44 μm) to obtain a wettable powder with an active ingredient of 60%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com