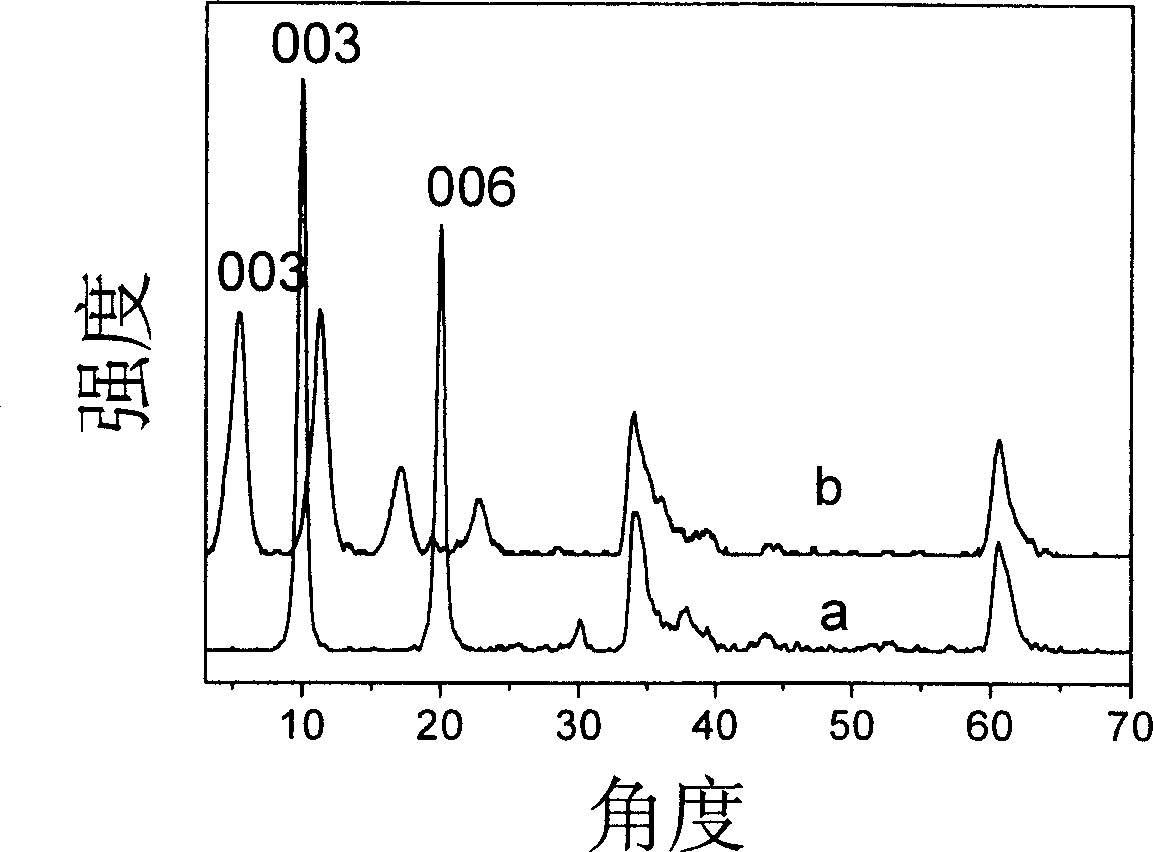
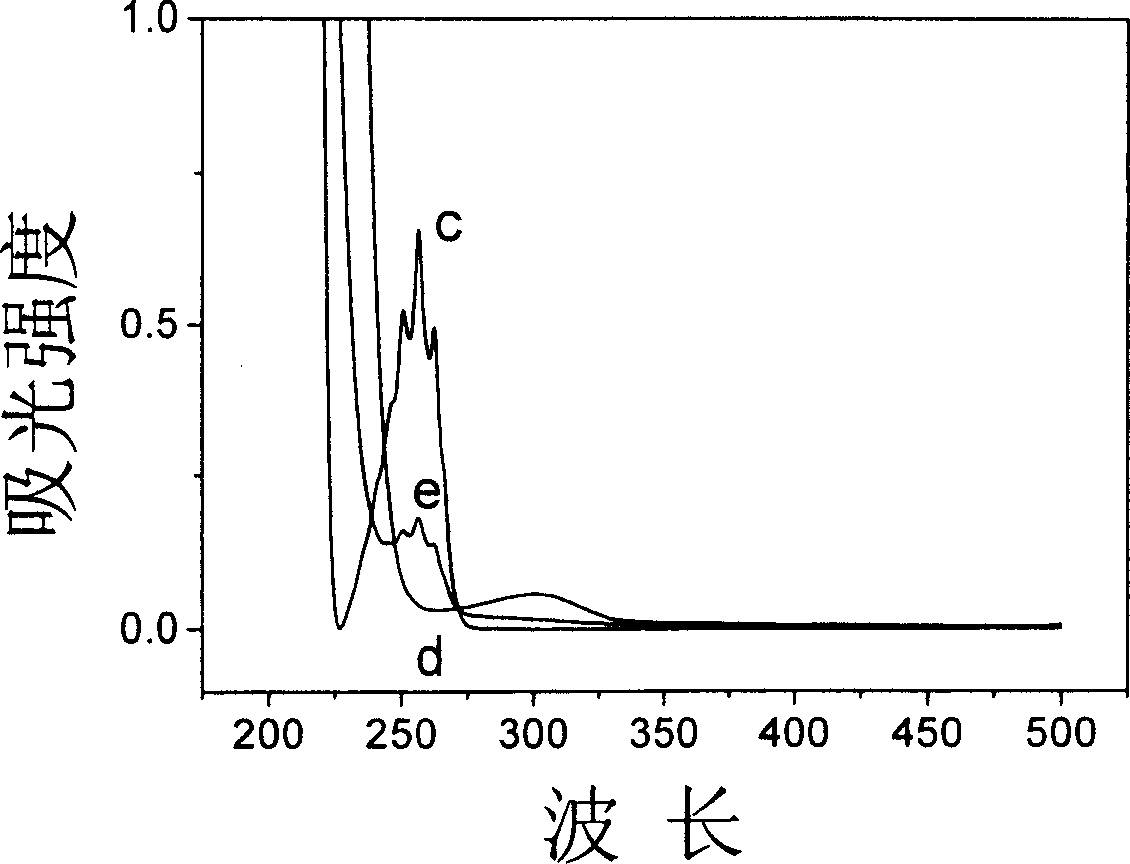
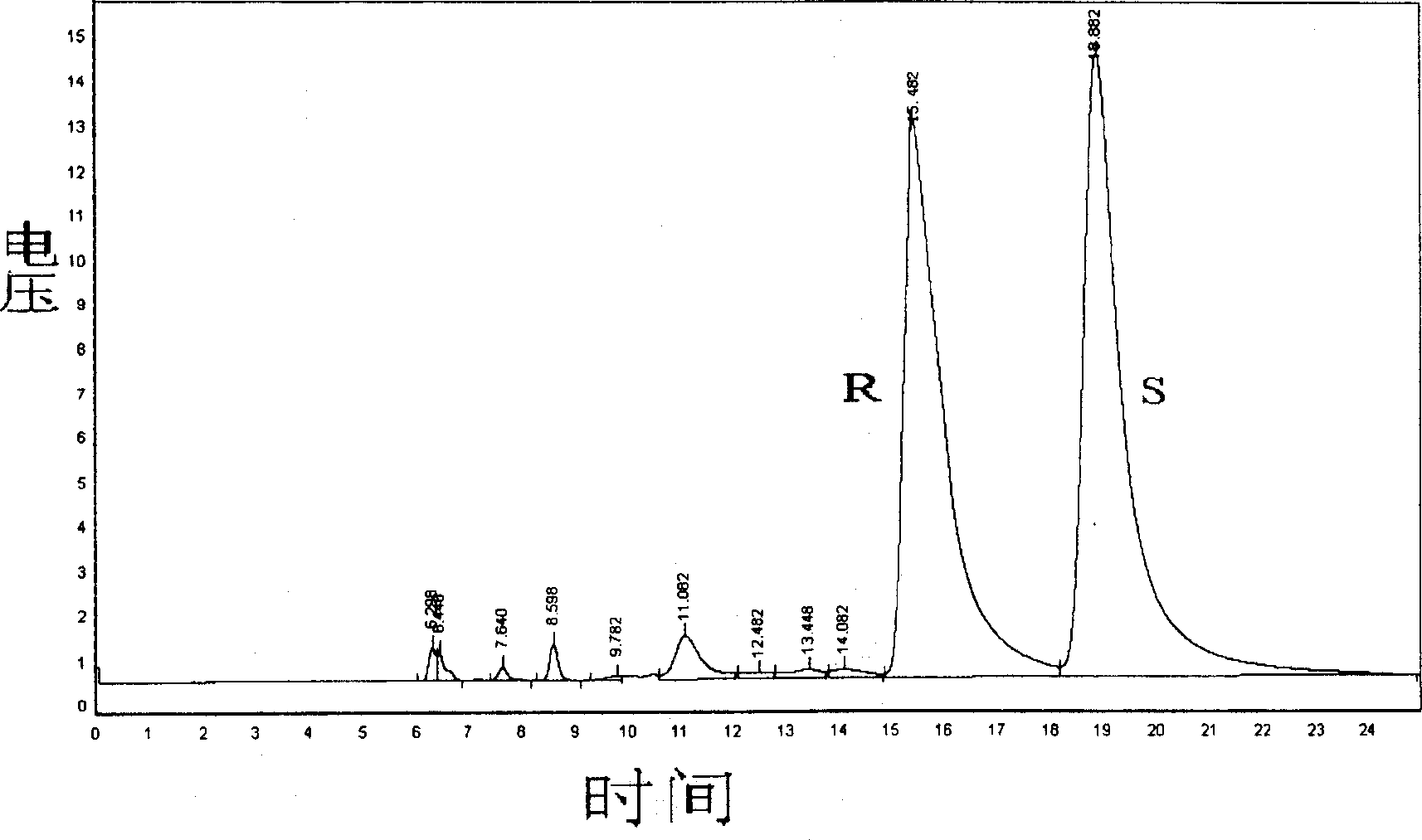
Carboxymethyl-beta-cyclodextrin intercalated water talc used for chiral resolution racemic modification and its preparation method
A racemate, chiral separation technology, applied in the direction of organic racemization, organic chemical methods, chemical instruments and methods, etc., can solve the problems of expensive columns, narrow application range, low performance, etc., to achieve easy reaction. , low cost, simple process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Step A: take by weighing 35.6g Zn(NO 3 ) 2 ·6H 2 O, 22.6g Al(NO 3 ) 3 9H 2 O and 9.06g NaNO 3 Dissolve in 200ml to remove CO 2 Mixed salt solution was prepared with water, another 11.2g NaOH was dissolved in 100ml to remove CO 2 Prepare alkaline solution in water, N at room temperature 2 Protection Use the double-drop method to slowly add the salt solution and alkali solution into the four-necked flask, and stir vigorously. Adjust the pH value to 7 with 0.1mol / L NaOH solution. The resulting slurry was crystallized at 70°C for 24 hours, and the product was centrifugally washed until neutral; the sample was taken out and dried at 70°C for 20 hours for characterization, and Zn 2 Al-NO 3 LDHs, whose Zn 2+ / Al 3+ =2.
[0028] Step B: 20.6 g of the sodium salt of carboxymethyl-β-cyclodextrin [C42 h 70-n o 35 ·(C 2 h 2 o 2 Na) n ] The solid was added into the four-neck flask containing the hydrotalcite precursor, N 2 Protected and heated to 70°C, reacted f...
Embodiment 2
[0032] Step A: take by weighing 30.7g Mg(NO 3 ) 2 ·6H 2 O and 22.6g Al(NO 3 ) 3 9H 2 O dissolved in 200ml to remove CO 2 Mixed salt solution was prepared with water, another 11.2g NaOH was dissolved in 100ml to remove CO 2 Prepare alkaline solution in water, N at room temperature 2 The salt solution and the alkali solution were slowly added into the four-necked flask by the double drop method, and the pH value was adjusted to 10 with 0.1mol / L NaOH solution after the dripping, and the resulting slurry was crystallized at 70°C for 24 hours, and the product was centrifugally washed until neutral; A small amount of sample was taken out and dried at 70°C for 20 hours for characterization, and Mg 2 Al-NO 3 LDHs, its Mg 2+ / Al 3+ =2.
[0033] Step B: According to the method similar to Step B in Example 1, the sodium salt of 20.6g carboxymethyl-β-cyclodextrin [C 42 h 70-n o 35 ·(C 2 h 2 o 2 Na) n ] The solid was added into the four-neck flask containing the sample, ...
Embodiment 3
[0037] Step A: Weigh 16.4g ZnCl 2 and 14.5g AlCl 3 ·6H 2 O dissolved in 200ml to remove CO 2 Mixed salt solution was prepared with water, another 11.2g NaOH was dissolved in 100ml to remove CO 2 Prepare alkaline solution in water, N at room temperature 2 The salt solution and the alkali solution were slowly added into the four-neck flask by the double-drop method, and the pH value was adjusted to 6 with 0.1mol / L NaOH solution after the dripping, and the obtained slurry was crystallized at 70°C for 24 hours, and the product was centrifugally washed until neutral; A small amount of samples were taken out and dried at 70°C for 20 hours for characterization, and Zn 2 Al-Cl LDHs, its Zn 2+ / Al 3+ =2.
[0038] Step B: According to the method similar to Step B in Example 1, the sodium salt of 20.6g carboxymethyl-β-cyclodextrin [C 42 h 70-n o 35 ·(C 2 h 2 o 2 Na) n ] The solid was added into the four-neck flask containing the sample, N 2 Protected and heated to 70°C for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com