Preparation method for synthesizing polyphenol compound by using enzyme immobilization technology
A technology for immobilizing polyphenols and enzymes, applied in biochemical equipment and methods, immobilizing enzymes, immobilizing on/in organic carriers, etc., can solve the problem of low yield of polyphenols, and achieve The effect of inhibiting the formation of quinones, high catalytic performance, and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
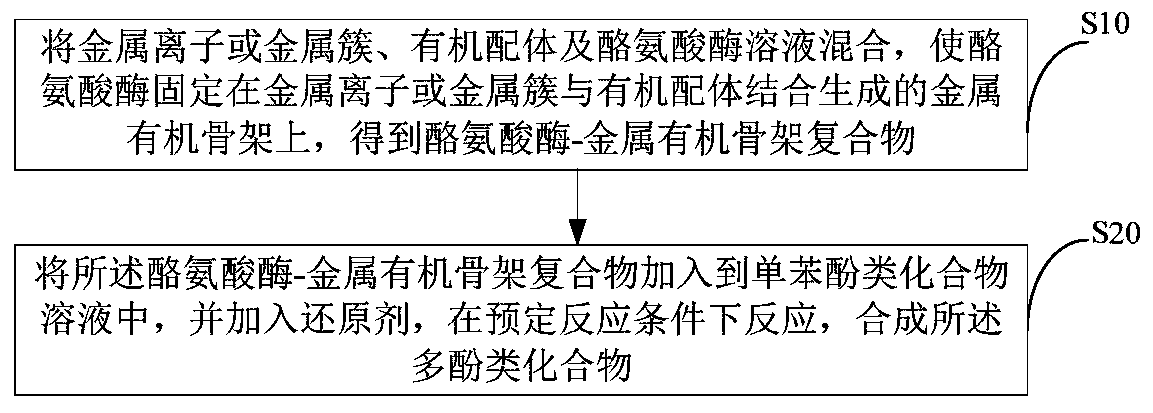
[0033] see figure 1 , figure 1 A flow chart of a preferred embodiment of a preparation method for synthesizing polyphenolic compounds with enzyme immobilization technology provided by the present invention, as shown in the figure, it includes the following steps:
[0034] S10, mixing metal ions or metal clusters, organic ligands and tyrosinase solution, immobilizing tyrosinase on the metal-organic framework formed by combining metal ions or metal clusters with organic ligands to obtain tyrosinase- metal-organic framework complexes;
[0035] S20, adding the tyrosinase-metal-organic framework complex into the monophenolic compound solution, and adding a reducing agent, and reacting under predetermined reaction conditions to synthesize the polyphenolic compound.
[0036] Through the synthesis method provided in this example, better immobilization effect and catalytic performance of tyrosinase can be obtained, and the synthesis method is simple to operate and can effectively inc...
Embodiment 1
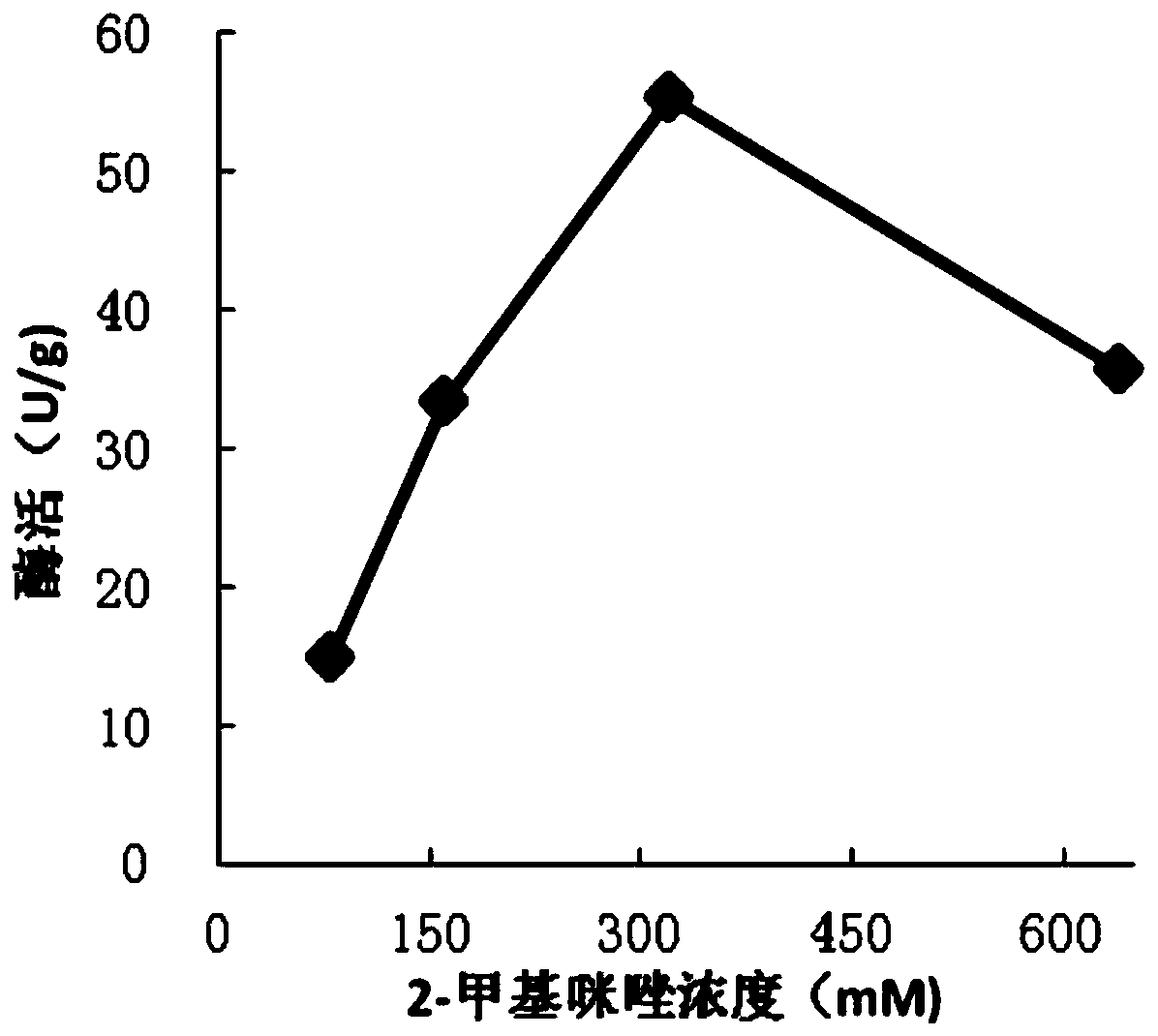
[0056] The effect of different conditions on the activity of TYR@ZIF-8.
[0057] To explore the effect of the concentration of 2-methylimidazole on the activity of the generated TYR@ZIF-8: prepare a total system of 200mL, 100mL tyrosinase solution, 80mL 2-methylimidazole solution of different concentrations and 20mL zinc acetate solution (0.4 M) Mix, stir at 4°C for 1 h, centrifuge the mixture (4°C, 8000 rpm, 10 min) and wash the precipitate several times with phosphate buffer (50 mM, pH 6.0), dry the precipitate in vacuum, and grind the sample to powder. Wherein the final concentration of 2-methylimidazole is 80mM, 160mM, 320mM, 640mM respectively, and the final concentration of zinc acetate is 40mM. The optimal concentration of 2-methylimidazole was obtained by measuring the enzyme activity of TYR@ZIF-8, wherein the method of enzyme activity determination was L-dopa as substrate, and the absorbance value at A475nm was measured by UV-Vis spectrophotometer , then according to...
Embodiment 2
[0062] HPLC detection spectrum of picatanol.
[0063] Shimadzu high performance liquid chromatograph, adopt reverse C18 post (Japan GLSciences, Inc company, InertsilODS-SP, 4.6 * 150mm, 5 μ m), this liquid condition is: mobile phase A is 0.5% acetic acid / acetonitrile=95 / 5 (v / v), the mobile phase B is acetonitrile / 0.5% acetic acid=95 / 5(v / v), A / B=75 / 25(v / v), the injection volume is 10μL, and the total flow rate is 1.0mL / min , the detection wavelength is 320nm, and the column temperature is 29°C.
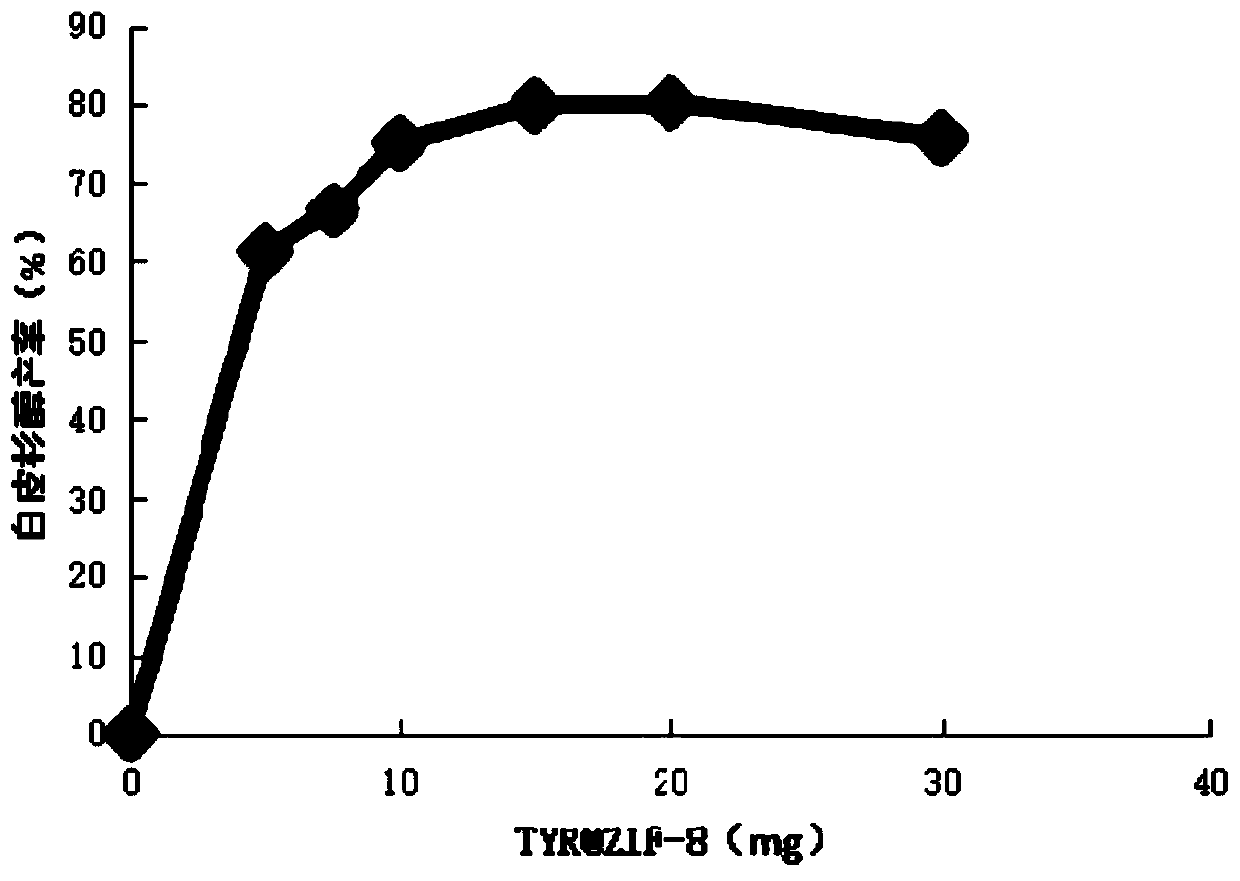
[0064] Using 50% acetonitrile (v / v) to accurately prepare picetanol standard solutions with serial concentrations, and detect the peak pattern of picetanol by HPLC. The picatatanol produced by TYR@ZIF-8 catalysis was properly diluted and analyzed qualitatively and quantitatively by HPLC. The HPLC detection spectrum of picatanol is as follows image 3 .
[0065] Such as image 3 As shown, the peak time of the product produced by TYR@ZIF-8 catalyzing resveratrol was consistent with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com