Rare-earth catalyst for diolefin polymerization and its prepn.
A rare earth catalyst and diolefin technology, applied in the field of rare earth catalyst and preparation, can solve the problems of difficult control and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
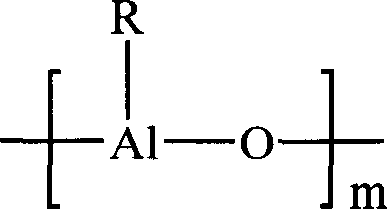
[0024] Under the protection of nitrogen, add 3.0×10 -4 mol of NdCl 3 3 (i-PrOH), 6 mL of 1.0 mol / L isoprene hexane solution, 4.5 mL of 2.0 mol / L MMAO toluene solution, and 9.5 mL of hexane was added. React at 20°C for 0.5 hour to obtain a rare earth catalyst for diolefin polymerization.
[0025] Under the protection of nitrogen, 2560 mL of hexane, 440 mL of isoprene (300 g) and the catalyst prepared above were added to a 5 L dry and deoxygenated autoclave. At this time, the monomer concentration is 10g / 100mL, and the Nd / isoprene ratio is 1.0×10 -6 mol / g. After reacting at 50°C for 4 hours, terminate the polymerization with an ethanol solution containing 1.0% 2,6-di-tert-butyl-p-cresol, then precipitate the polymer in excess ethanol, wash and extrude it with ethanol, and place it at 40°C Drying under reduced pressure for 24 hours yielded 285 g of isoprene polymerization product with a conversion rate of 95%. The cis-1,4 structure content measured by infrared spectrum is 96...
Embodiment 2
[0027] As in the catalyst preparation method and polymerization conditions in Example 1, only with NdCl 3 3EtOH instead of NdCl 3 • 3(i-PrOH).
[0028] The results of the obtained polyisoprene are as follows: the polymerization product is 240g, the conversion rate is 80%, the cis-1 and 4 structure content is 95.6% as measured by infrared spectroscopy, and the number average molecular weight is 146200 as measured by GPC. is 1.85.
Embodiment 3
[0030] As in the catalyst preparation method and polymerization conditions in Example 1, only with NdCl 3 ·3CH 3 (CH 2 ) 5 OH instead of NdCl 3 • 3(i-PrOH).
[0031] At this time, the result of using the polyisoprene obtained therefrom is as follows: 270 g of the polymerized product, the conversion rate is 90%, the cis-1, 4 structure content is 96.0% as measured by infrared spectroscopy, and the number average molecular weight as measured by GPC is 189,500. The molecular weight distribution was 1.83.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com