Solid polymer fuel cell
A solid electrolyte and fuel cell technology, applied in solid electrolyte fuel cells, fuel cells, fuel cell components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
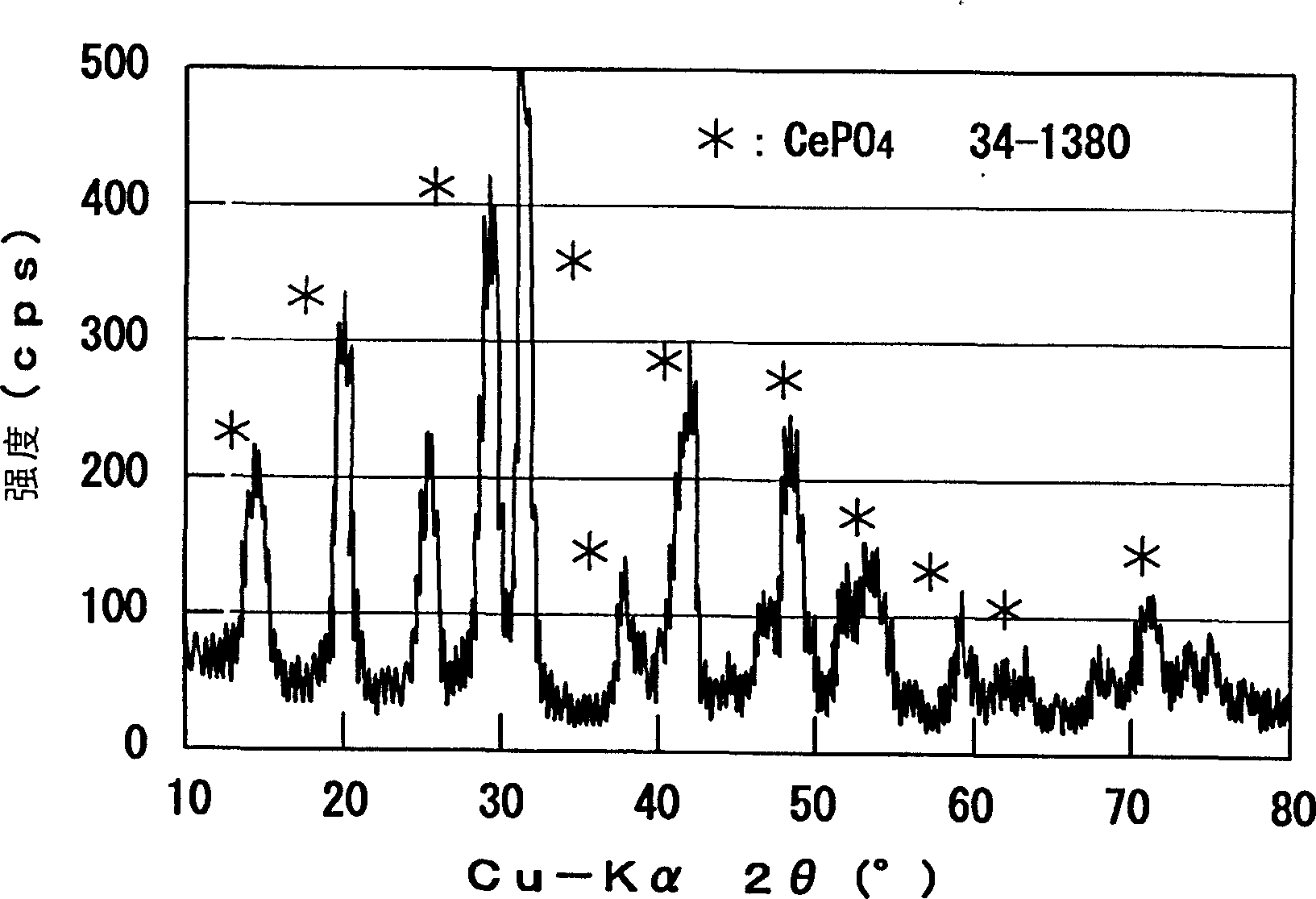
[0081] By adding cerium nitrate (Ce(NO 3 ) 3 ·6H 2 O) was added to 100 ml of water to prepare an aqueous solution so that the concentration of Ce ions was 0.05 wt%. A perfluorinated electrolyte film (Nafion (registered trademark) film) was immersed in this aqueous solution, and heated at 90° C. for 2 hours. Then, the film was immersed in 100ml of 0.1M H 3 PO 4 aqueous solution, and hydrolyzed at 90°C for 1 hour. The color of the film gradually changed to white, and Ce phosphate was fixed to the inside of the film. Then, the membrane was washed several times with ion-exchanged water and heated at 90 °C for 30 min in ion-exchanged water to remove excess H 3 PO 4 . The fixed amount of Ce phosphate obtained from the weight change of the film was 1.6 wt%.
[0082] Next, this film was placed in 200ml of hydrogen peroxide and iron (II) chloride (Fe 2 Cl) (corresponding to 14 ppm of Fe) in an aqueous solution, and subjected to an immersion test at 100°C for 24 hours. After ...
example 2
[0084] The perfluorinated electrolyte film with Gd phosphate immobilized on it was prepared by the same process as in Example 1, except that gadolinium nitrate (Gd(NO 3 ) 3 ) as a water-soluble salt. The fixed amount of phosphoric acid Gd obtained from the weight change of the film was 4.3 wt%. For the obtained film, F ion concentration and weight loss were measured under the same conditions as in Example 1. As a result, the concentration of F ions was 18.4 ppm and the weight loss was -1.3 wt%.
example 3
[0086] The perfluorinated electrolyte film on which La phosphate was immobilized was prepared by the same process as in Example 1, except that lanthanum nitrate (La(NO 3 ) 3 ) as a water-soluble salt. The fixed amount of phosphoric acid La obtained from the weight change of the film was 3.9 wt%. For the obtained film, the F ion concentration was measured under the same conditions as in Example 1. As a result, the concentration of F ions was 5.8 ppm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com