Ramatroban cyclodextrin inclusion
A technology of ramatroban and cyclodextrin, which is applied in the field of ramatroban pharmaceutical preparations, can solve problems such as unfavorable practical application, industrial production, changes in bioavailability, etc., and achieves improved bioavailability, simple method, and increased stability. sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 5 g of β-cyclodextrin and 1 gram of ramatroban were reacted in 25 ml of water-ethanol (1:2) mixture for 12 hours at room temperature. The solvent was removed by spray-drying or freeze-drying to obtain a white microcrystalline solid, which was shown to be a new crystalline inclusion by X-ray powder diffraction. The inclusions are suitable for direct compression or filled capsules.
Embodiment 2
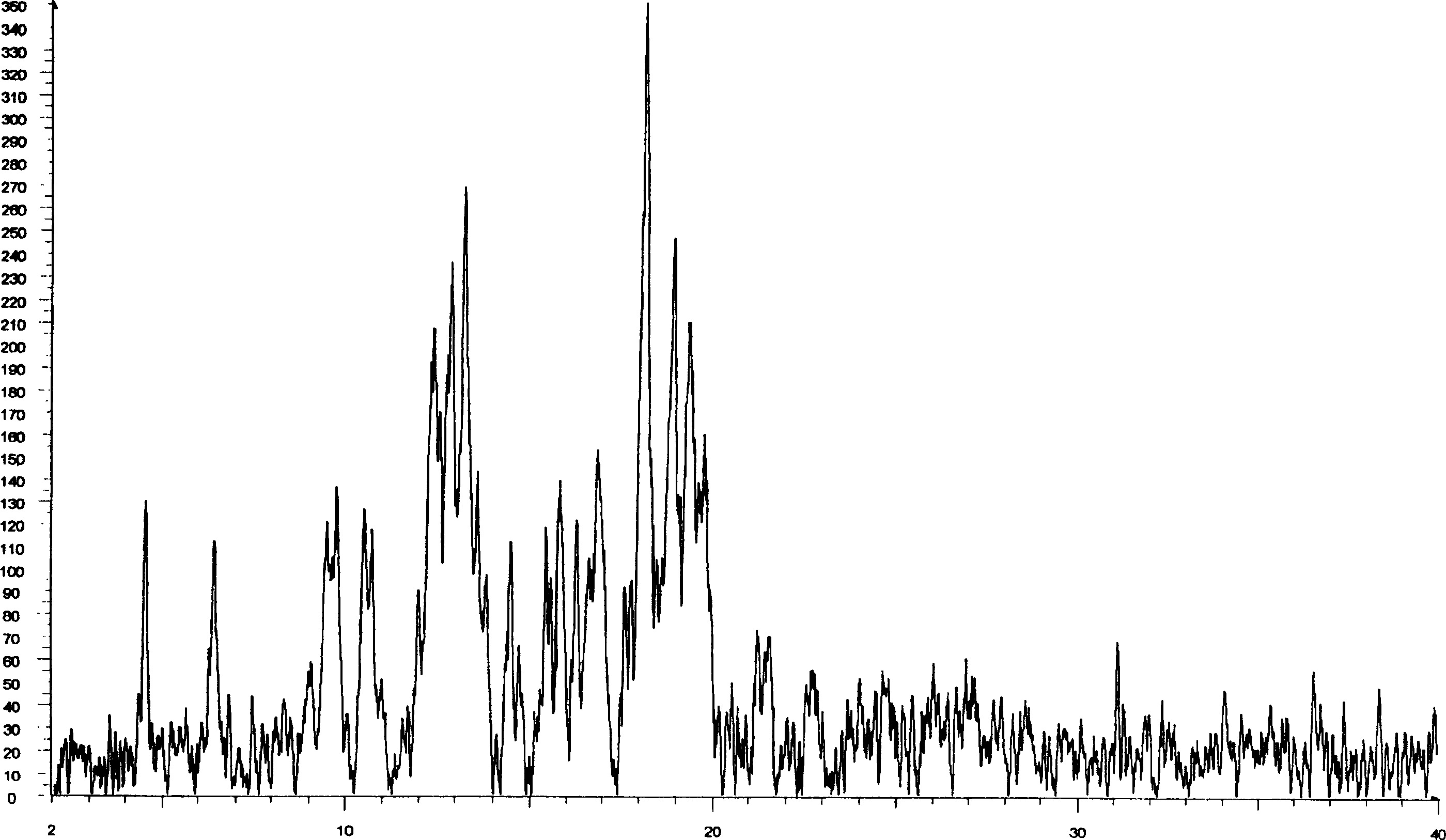
[0020] Add 5 grams of ramatroban, add appropriate amount of ethanol to dissolve it, weigh another 5 grams of hydroxypropyl cyclodextrin, add water, and make a saturated solution in a constant temperature water bath at 40°C. Under electric stirring, slowly add the ramatroban ethanol solution into the hydroxypropyl cyclodextrin solution, stir at constant temperature for 1 hour, stop heating, and continue stirring to room temperature to obtain a white suspension, put it in the refrigerator for 12 hours, pump Filter, and vacuum-dry the precipitate at 40°C, pass through an 80-mesh sieve, and dry it for later use. X-ray powder diffraction showed that it was an inclusion of a new crystalline state (see figure 1 ). The inclusions can be directly compressed into tablets or filled into capsules.
Embodiment 3
[0022] Add 5 grams of β-cyclodextrin and 25ml of water, grind it evenly with a high-energy grinder, add 5 grams of Rematroban, and grind it fully until it becomes a paste, about 1 hour, after drying in a freeze dryer for 4 hours, use 10ml Rinse the dry matter with ethanol, and then freeze-dry it.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com