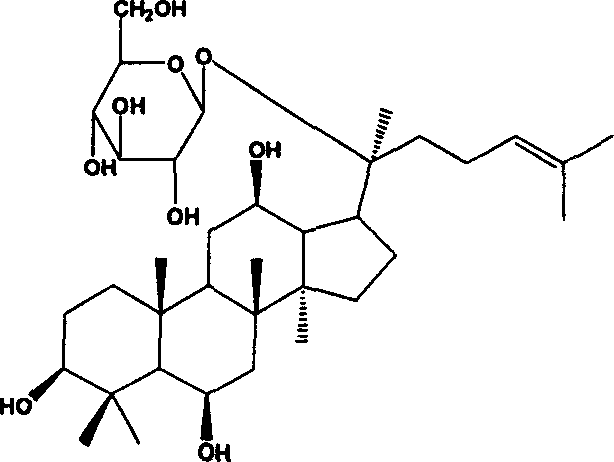
Ginsenoside F1 medicinal uses
A technology of ginsenosides and drugs, applied in the field of medicine, can solve problems that have not been reported, and achieve the effect of small side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0073] Preparation Example 1 Ginsenoside F 1preparation of
[0074] 500mg ginsenoside Re and 150mg naringinase were dissolved in 50ml of phosphoric acid-citric acid buffer (pH 4.5, ionic strength 0.01M, containing 10% ethanol), hydrolyzed in a water bath at 40°C for 7 days; the reaction solution was centrifuged Collect the precipitate; wash the precipitate with 10ml of water, wash 3 times (10ml×3), combine the washing liquid with the mother liquor, concentrate to 50ml for reuse; wash the precipitate with 10ml ethanol, wash 5 times (10ml×5), combine the ethanol solution, Evaporate to dryness and collect the residue to obtain ginsenoside F 1 301 mg. Determination of Ginsenoside F by HPLC 1 The content is 63%.
preparation example 2
[0075] Preparation Example 2 Ginsenoside F 1 preparation of
[0076] 500mg Ginsenoside Rg 1 , 150mg of naringinase was dissolved in 50ml of glycine-HCl buffer (pH 4.5, ionic strength 0.01M, containing 10% dimethyl sulfoxide), and hydrolyzed in a water bath at 40°C for 7 days; the reaction solution was centrifuged to collect the precipitate ; Wash the precipitate with 10ml of water, wash 3 times (10ml × 3), combine the lotion with the mother liquor, concentrate to 50ml for reuse; wash the precipitate with 10ml of ethanol, wash 5 times (10ml × 5), combine the ethanol solution, evaporate to dryness , collect the residue to get ginsenoside F 1 325mg. Determination of Ginsenoside F by HPLC 1 The content is 84%.
preparation example 3
[0077] Preparation Example 3 Ginsenoside F 1 preparation of
[0078] 500mg Ginsenosides Re and Rg 1 The mixture, 150mg of naringinase was dissolved in 50ml of acetic acid-sodium acetate buffer solution (pH 4.5, ionic strength of 0.01M, containing 10% formamide), and hydrolyzed in a water bath at 40°C for 7 days; the reaction solution was collected by centrifugation Precipitate; wash the precipitate with 10ml of water, wash 3 times (10ml×3), combine the lotion with the mother liquor, concentrate to 50ml for reuse; wash the precipitate with 10ml ethanol, wash 5 times (10ml×5), combine the ethanol solution, distill Dry and collect the residue to get ginsenoside F 1 310mg. Determination of Ginsenoside F by HPLC 1 The content is 69%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com